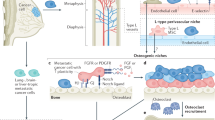
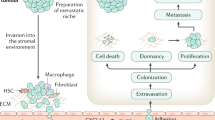
Abstract
Bone metastases are a frequent and severe complication of advanced-stage cancers. Breast and prostate cancers, the most common malignancies in women and men, respectively, have a particularly high propensity to metastasize to bone. Conceptually, circulating tumour cells (CTCs) in the bloodstream and disseminated tumour cells (DTCs) in the bone marrow provide a snapshot of the dissemination and colonization process en route to clinically apparent bone metastases. Many cell types that constitute the bone microenvironment, including osteoblasts, osteocytes, osteoclasts, adipocytes, endothelial cells, haematopoietic stem cells and immune cells, engage in a dialogue with tumour cells. Some of these cells modify tumour biology, while others are disrupted and out-competed by tumour cells, thus leading to distinct phases of tumour cell migration, dormancy and latency, and therapy resistance and progression to overt bone metastases. Several current bone-protective therapies act by interrupting these interactions, mainly by targeting tumour cell–osteoclast interactions. In this Review, we describe the functional roles of the bone microenvironment and its components in the initiation and propagation of skeletal metastases, outline the biology and clinical relevance of CTCs and DTCs, and discuss established and future therapeutic approaches that specifically target defined components of the bone microenvironment to prevent or treat skeletal metastases.
Key points
-
Bone metastases are frequent events associated with advanced-stage malignancies, particularly breast and prostate cancers, and often result in pathological fractures, pain, disability, reduced quality of life and a poor prognosis.
-
Circulating tumour cells can be detected in the blood using standardized liquid biopsy assays and can provide insights into the metastatic process, inform clinical risk stratification, and enable monitoring and tracing of resistance to therapy.
-
Disseminated tumour cells (DTCs) can be detected in the bone marrow through bone marrow aspiration. Their fate is variable and can include apoptosis or immune-mediated cell death, persistence and dormancy, or progression to overt bone metastases.
-
DTCs mutually interact with diverse components of the bone microenvironment, including bone cells, adipocytes, endothelial cells and various immune cells as well as the extracellular matrix. Survival strategies of DTCs involve interference with bone cell and adipocyte functions, immune evasion and neoangiogenesis.
-
On the basis of emerging knowledge of the biology of bone metastasis, several bone-targeted therapies are currently under evaluation in preclinical studies and clinical trials.
-
Approved therapies for patients with established bone metastases include bisphosphonates, the anti-receptor activator of NF-κB ligand (RANKL) antibody denosumab and radium-223.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Anderson, R. L. et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 16, 185–204 (2019).
Sartor, O. et al. Metastatic prostate cancer. N. Engl. J. Med. 378, 645–657 (2018).
Hofbauer, L. C. et al. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2, 500–512 (2014).
Bubendorf, L. et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 (2000).
Lee, Y. T. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 23, 175–180 (1983).
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Xiao, W. et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag. Res. 10, 5329–5338 (2018).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Prim. 6, 83 (2020).
Croucher, P. I. et al. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16, 373–386 (2016).
Gori, F. et al. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 141, 4768–4776 (2000).
Onishi, T. et al. Future directions of bone-targeted therapy for metastatic breast cancer. Nat. Rev. Clin. Oncol. 7, 641–651 (2010).
Dallas, S. L. et al. The osteocyte: an endocrine cell and more. Endocr. Rev. 34, 658–690 (2013).
Luo, Y. et al. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab. 22, 886–894 (2015).
Mohme, M. et al. Circulating and disseminated tumour cells – mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14, 155–167 (2017).
Pantel, K. et al. Liquid biopsy and minimal residual disease – latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 (2019).
Mazel, M. et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782 (2015).
Rachner, T. D. et al. Prognostic value of RANKL/OPG serum levels and disseminated tumor cells in nonmetastatic breast cancer. Clin. Cancer Res. 25, 1369–1378 (2019).
Bianchini, G. et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016).
Stopeck, A. T. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 28, 5132–5139 (2010).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Smith, M. R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012).
Van Poznak, C. et al. Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario focused guideline update. J. Clin. Oncol. 35, 3978–3986 (2017).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Kollermann, J. et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J. Clin. Oncol. 26, 4928–4933 (2008).
Magbanua, M. J. M. et al. Genomic and expression profiling reveal molecular heterogeneity of disseminated tumor cells in bone marrow of early breast cancer. NPJ Breast Cancer 4, 31 (2018).
Stoecklein, N. H. & Klein, C. A. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int. J. Cancer 126, 589–598 (2010).
Fehm, T. et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412 (2010).
Riethdorf, S. et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645 (2010).
Jordan, N. V. et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537, 102–106 (2016).
Brabletz, T., Kalluri, R., Nieto, M. A. & Weinberg, R. A. EMT in cancer. Nat. Rev. Cancer 18, 128–134 (2018).
Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl Cancer Inst. 110, 560–567 (2018).
Kuske, A. et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 6, 39736 (2016).
Watson, M. A. et al. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin. Cancer Res. 13, 5001–5009 (2007).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Werner, S. et al. Suppression of early hematogenous dissemination of human breast cancer cells to bone marrow by retinoic acid-induced 2. Cancer Discov. 5, 506–519 (2015).
Pantel, K. et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl Cancer Inst. 85, 1419–1424 (1993).
Braun, S. et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J. Clin. Oncol. 18, 80–86 (2000).
Celia-Terrassa, T. & Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 20, 868–877 (2018).
LeBleu, V. S. et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003 (2014).
Bartkowiak, K. et al. Disseminated tumor cells persist in the bone marrow of breast cancer patients through sustained activation of the unfolded protein response. Cancer Res. 75, 5367–5377 (2015).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Ell, B. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–556 (2013).
Bednarz-Knoll, N. et al. Potential involvement of Jagged1 in metastatic progression of human breast carcinomas. Clin. Chem. 62, 378–386 (2016).
Zhang, D. et al. Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int. 17, 6 (2017).
Gawrzak, S. et al. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+ breast cancer. Nat. Cell Biol. 20, 211–221 (2018). Erratum in: Nat Cell Biol. 20, 990 (2018).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Taichman, R. S. et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8, e61873 (2013).
Shiozawa, Y. et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 12, 116–127 (2010).
Bragado, P. et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351–1361 (2013).
Yu-Lee, L. Y. et al. Osteoblast-secreted factors mediate dormancy of metastatic prostate cancer in the bone via activation of the TGFβRIII-p38MAPK-pS249/T252RB pathway. Cancer Res. 78, 2911–2924 (2018).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208, 2641–2655 (2011).
Cackowski, F. C. et al. Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. J. Cell Biochem. 118, 891–902 (2017).
Naume, B. et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857 (2014).
Riethdorf, S. et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “Geparquattro” trial. Clin. Cancer Res. 23, 5384–5393 (2017).
Keller, L. & Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 19, 553–567 (2019).
Alix-Panabieres, C. et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin. Chem. 53, 537–539 (2007).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Koch, C. et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 12, e11908 (2020).
Faugeroux, V. et al. Genetic characterization of a unique neuroendocrine transdifferentiation prostate circulating tumor cell-derived eXplant model. Nat. Commun. 11, 1884 (2020).
Tjensvoll, K. et al. Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer 19, 1131 (2019).
Decker, A. M., Jung, Y., Cackowski, F. & Taichman, R. S. The role of hematopoietic stem cell niche in prostate cancer bone metastasis. J. Bone Oncol. 5, 117–120 (2016).
Allocca, G. et al. The bone metastasis niche in breast cancer-potential overlap with the haematopoietic stem cell niche in vivo. J. Bone Oncol. 17, 100244 (2019).
Janni, W. et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse–a European pooled analysis. Clin. Cancer Res. 17, 2967–2976 (2011).
Bidard, F. C., Proudhon, C. & Pierga, J. Y. Circulating tumor cells in breast cancer. Mol. Oncol. 10, 418–430 (2016).
Trapp, E. et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J. Natl Cancer Inst. 111, 380–387 (2019).
Sparano, J. et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 4, 1700–1706 (2018).
Bidard, F.-C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414 (2014).
Franken, A. et al. Detection of ESR1 mutations in single circulating tumor cells on estrogen deprivation therapy but not in primary tumors from metastatic luminal breast cancer patients. J. Mol. Diagn. 22, 111–121 (2020).
Gao, J. J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21, 250–260 (2020).
Pfitzenmaier, J. et al. The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol. Oncol. 25, 214–220 (2007).
Pantel, K., Hille, C. & Scher, H. I. Circulating tumor cells in prostate cancer: from discovery to clinical utility. Clin. Chem. 65, 87–99 (2019).
Danila, D. C. et al. Clinical validity of detecting circulating tumor cells by AdnaTest assay compared with direct detection of tumor mRNA in stabilized whole blood, as a biomarker predicting overall survival for metastatic castration-resistant prostate cancer patients. Cancer J. 22, 315–320 (2016).
Lorente, D. et al. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur. Urol. 70, 985–992 (2016).
Heller, G. et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-cpecific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 36, 572–580 (2018).
Kirby, M., Hirst, C. & Crawford, E. D. Characterising the castration-resistant prostate cancer population: a systematic review. Int. J. Clin. Pract. 65, 1180–1192 (2011).
Vlachostergios, P. J., Puca, L. & Beltran, H. Emerging variants of castration-resistant prostate cancer. Curr. Oncol. Rep. 19, 32 (2017).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Antonarakis, E. S. et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 35, 2149–2156 (2017).
Armstrong, A. J. et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J. Clin. Oncol. 37, 1120–1129 (2019).
Scher, H. I. et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 4, 1179–1186 (2018).
Sharp, A. et al. Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur. Urol. 76, 676–685 (2019).
Hille, C. et al. Detection of androgen receptor variant 7 (ARV7) mRNA levels in EpCAM-enriched CTC fractions for monitoring response to androgen targeting therapies in prostate cancer. Cells 8, 1067 (2019).
Gorges, T. M. et al. Heterogeneous PSMA expression on circulating tumor cells: a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget 7, 34930–34941 (2016).
Autio, K. A. et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 4, 1344–1351 (2018).
Maurer, T. et al. PSMA theranostics using PET and subsequent radioguided surgery in recurrent prostate cancer. Clin. Genitourin. Cancer 14, e549–e552 (2016).
Schindlbeck, C. et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. J. Cancer Res. Clin. Oncol. 139, 1055–1062 (2013).
Magbanua, M. J. M. et al. Synchronous detection of circulating tumor cells in blood and disseminated tumor cells in bone marrow predicts adverse outcome in early breast cancer. Clin. Cancer Res. 25, 5388–5397 (2019).
Pak, S. et al. Association between postoperative detection of circulating tumor cells and recurrence in patients with prostate cancer. J. Urol. 203, 1128–1134 (2020).
Broncy, L. & Paterlini-Bréchot, P. Clinical impact of circulating tumor cells in patients with localized prostate cancer. Cells 8, 676 (2019).
Cristofanilli, M. et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit. Rev. Oncol. Hematol. 134, 39–45 (2019).
Giuliano, M. et al. Circulating and disseminated tumor cells from breast cancer patient-derived xenograft-bearing mice as a novel model to study metastasis. Breast Cancer Res. 17, 3 (2015).
Bianco, P. et al. Skeletal stem cells. Development 142, 1023–1027 (2015).
Chan, C. K. F. et al. Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298 (2015).
Lowery, J. W. et al. The BMP pathway and its inhibitors in the skeleton. Physiol. Rev. 98, 2431–2452 (2018).
Kamizaki, K. et al. Role of noncanonical Wnt ligands and Ror-family receptor tyrosine kinases in the development, regeneration, and diseases of the musculoskeletal system. Dev. Dyn. 250, 27–38 (2021).
Zieba, J. T. et al. Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules 10, 332 (2020).
Ziouti, F. et al. NOTCH signaling is activated through mechanical strain in human bone marrow-derived mesenchymal stromal cells. Stem Cells Int. 2019, 5150634 (2019).
Herrmann, M. et al. Interactions between muscle and bone–where physics meets biology. Biomolecules 10, 432 (2020).
Ganesh, T. et al. Multiscale finite element modeling of mechanical strains and fluid flow in osteocyte lacunocanalicular system. Bone 137, 115328 (2020).
Jacome-Galarza, C. E. et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 568, 541–545 (2019).
Cappariello, A. et al. The great beauty of the osteoclast. Arch. Biochem. Biophys. 558, 70–78 (2014).
Tsukasaki, M. et al. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–664 (2019).
Wortzel, I. et al. Exosome-mediated metastasis: communication from a distance. Dev. Cell 49, 347–360 (2019).
Paiva, A. E. et al. Pericytes in the pre-metastatic niche. Cancer Res. 78, 2779–2786 (2018).
Schramek, D. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010).
Reyes, M. E. et al. Poor prognosis of patients with triple-negative breast cancer can be stratified by RANK and RANKL dual expression. Breast Cancer Res. Treat. 164, 57–67 (2017).
Geerts, D. et al. Osteoprotegerin: relationship to breast cancer risk and prognosis. Front. Oncol. 10, 462 (2020).
Das, S. et al. The CaSR in pathogenesis of breast cancer: a new target for early stage bone metastases. Front. Oncol. 10, 69 (2020).
Wu, X. et al. RANKL/RANK system-based mechanism for breast cancer bone metastasis and related therapeutic strategies. Front. Cell Dev. Biol. 8, 76 (2020).
Fazeli, P. K. et al. Marrow fat and bone–new perspectives. J. Clin. Endo Metab. 98, 935–945 (2013).
Lecka-Czernik, B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone 50, 534–539 (2012).
Montalvany-Antonucci, C. C. et al. High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J. Nutr. Biochem. 59, 93–103 (2018).
Evangelista, G. C. M. et al. 4T1 mammary carcinoma colonization of metastatic niches is accelerated by obesity. Front. Oncol. 9, 685 (2019).
Tsai, C. F. et al. Induction of osteoclast-like cell formation by leptin-induced soluble intercellular adhesion molecule secreted from cancer cells. Ther. Adv. Med. Oncol. 11, 1758835919846806 (2019).
Feng, H. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol. Res. 21, 165–171 (2013).
Templeton, Z. S. et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia 17, 849–861 (2015).
Tang, C. H. et al. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-κB pathways. Prostate 69, 1781–1789 (2009).
Sun, G. et al. The adiponectin-AdipoR1 axis mediates tumor progression and tyrosine kinase inhibitor resistance in metastatic renal cell carcinoma. Neoplasia 21, 921–931 (2019).
Wu, J. B. et al. MAOA-dependent activation of Shh-IL6-RANKL signaling network promotes prostate cancer metastasis by engaging tumor-stromal cell interactions. Cancer Cell 31, 368–382 (2017).
Chen, G. L. et al. High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6. Oncotarget 7, 26653–26669 (2016).
Hardaway, A. et al. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin. Exp. Metastasis 32, 353–368 (2015).
Wang, J. et al. Adipogenic niches for melanoma cell colonization and growth in bone marrow. Lab. Invest. 97, 737–745 (2017).
Diedrich, J. D. et al. The lipid side of bone marrow adipocytes: how tumor cells adapt and survive in bone. Curr. Osteoporos. Rep. 16, 443–457 (2018).
Diedrich, J. D. et al. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1α activation. Oncotarget 7, 64854–64877 (2016).
Singh, A. et al. Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI Insight 4, e125679 (2019).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Stucker, S., Chen, J., Watt, F. E. & Kusumbe, A. P. Bone angiogenesis and vascular niche remodeling in stress, aging, and diseases. Front. Cell Dev. Biol. 8, 602269 (2020).
Rafii, S. et al. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Supakul, S. et al. Pericytes as a source of osteogenic cells in bone fracture healing. Int. J. Mol. Sci. 20, 1079 (2019).
Diomede, F. et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 21, 3242 (2020).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Shan, M. et al. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl Sci. 162, 93–119 (2019).
Yue, L. et al. Fucosyltransferase 8 expression in breast cancer patients: a high throughput tissue microarray analysis. Histol. Histopathol. 31, 547–555 (2016).
do Nascimento, J. C., Beltrão, E. I. & Rocha, C. R. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj. J. 37, 263–275 (2020).
Esposito, M. et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 (2019).
Wang, X. et al. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology 24, 935–944 (2014).
Carrascal, M. A. et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol. Oncol. 12, 579–593 (2018).
Andonegui-Elguera, M. A. et al. An overview of vasculogenic mimicry in breast cancer. Front. Oncol. 10, 220 (2020).
Maiti, A. et al. Class I histone deacetylase inhibitor suppresses vasculogenic mimicry by enhancing the expression of tumor suppressor and anti-angiogenesis genes in aggressive human TNBC cells. Int. J. Oncol. 55, 116–130 (2019).
Li, S. et al. Inhibitory effects of compound DMBT on hypoxia-induced vasculogenic mimicry in human breast cancer. Biomed. Pharmacother. 96, 982–992 (2017).
Kuczynski, E. A. et al. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Geraets, S. E. W. et al. Preoperative embolization in surgical treatment of long bone metastasis: a systematic literature review. EFORT Open Rev. 5, 17–25 (2020).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animal. Nature 508, 269–273 (2014).
Knowles, H. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia 3, 73–82 (2015).
Devignes, C. S. et al. HIF signalling in osteoblast lineage cells promotes systemic breast cancer growth and mestastasis in mice. Proc. Natl Acad. Sci. USA 5, E992–E1001 (2018).
Tam, S. Y., Wu, V. W. & Law, H. K. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and beyond. Front. Oncol. 10, 486 (2020).
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D. & Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 (2013).
Mimeault, M. & Batra, S. K. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J. Cell Mol. Med. 17, 30–54 (2013).
Gu, J. et al. Hypoxia-induced up-regulation of angiopoietin-2 in colorectal cancer. Oncol. Rep. 15, 779–783 (2006).
Johnson, R. W. et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 18, 1078–1089 (2016). Erratum in: Nat Cell Biol. 18, 1260 (2016).
Laoui, D. et al. Tumor hypoxia does not drive differentiation of tumor associated macrophages but rather fine tunes the M2 like macrophage population. Cancer Res. 1, 24–30 (2014).
Huber, R. et al. Tumor hypoxia promotes melanoma growth and metastasis via HMGB1. Sci. Rep. 6, 29914 (2016).
Doedens, A. L. et al. Macrophages expression of HIF1a suppresses T cell function and promotes tumor progression. Cancer Res. 70, 7465–7475 (2010).
Mascanfroni, I. D. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 21, 638–646 (2015).
Kolb, A. D. et al. The bone extracellular matrix as an ideal milieu for cancer cell metastases. Cancers 11, 1020 (2019).
Maller, O. et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. https://doi.org/10.1038/s41563-020-00849-5 (2020).
Setargew, Y. F., Wyllie, K., Grant, R. D., Chitty, J. L. & Cox, T. R. Targeting lysyl oxidase family meditated matrix cross-linking as an anti-stromal therapy in solid tumours. Cancers 13, 491 (2021).
Lin, T. C. et al. Fibronectin in cancer: friend or foe. Cells 9, 27 (2019).
Sens, C. et al. Fibronectins containing extradomain A or B enhance osteoblast differentiation via distinct integrins. J. Biol. Chem. 292, 7745–7760 (2017).
Rossnagl, S. et al. EDA-fibronectin originating from osteoblasts inhibits the immune response against cancer. PLoS Biol. 14, e1002562 (2016).
Lieverse, R. I. Y. et al. Human fibronectin extra domain B as a biomarker for targeted therapy in cancer. Mol. Oncol. 14, 1555–1568 (2020).
Pang, X. et al. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 144, 235–244 (2019).
Kovacheva, M. et al. Conditional knockdown of osteopontin inhibits breast cancer skeletal metastasis. Int. J. Mol. Sci. 20, 4918 (2019).
Elgundi, Z. et al. Cancer metastasis: the role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front. Oncol. 9, 1482 (2020).
Li, Y. et al. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS ONE 14, e0218067 (2019).
Yang, Y. et al. Systemic delivery of an oncolytic adenovirus expressing decorin for the treatment of breast cancer bone metastases. Hum. Gene Ther. 26, 813–825 (2015).
Gubbiotti, M. A. et al. Proteoglycan signaling in tumor angiogenesis and endothelial cell autophagy. Semin. Cancer Biol. 62, 1–8 (2020).
Walimbe, T. et al. Proteoglycans in biomedicine: resurgence of an underexploited class of ECM molecules. Front. Pharmacol. 10, 1661 (2020).
Sinder, B. P., Pettit, A. R. & McCauley, L. K. Macrophages: their emerging roles in bone. J. Bone Miner. Res. 30, 2140–2149 (2015).
Wang, Y. et al. MDSCs: key criminals of tumor pre-metastatic niche formation. Front. Immunol. 10, 172 (2019).
Danilin, S. et al. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 1, 1484–1494 (2012).
D'Amico, L. et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 213, 827–840 (2016).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004).
Soki, F. N. et al. Bone marrow macrophages support prostate cancer growth in bone. Oncotarget 6, 35782–35796 (2015).
Sullivan, A. R. et al. CSF-1R signaling in health and disease: a focus on the mammary gland. J. Mammary Gland. Biol. Neoplasia. 19, 149–159 (2014).
Bendell, J. C. et al. A phase 1 study of ARRY-382, an oral inhibitor of colony-stimulating factor-1 receptor (CSF1R), in patients with advanced or metastatic cancers [abstract]. Mol. Cancer Ther. 12 (Suppl. 11), A252 (2013).
Jones, J. D. et al. Trabectedin reduces skeletal prostate cancer tumor size in association with effects on M2 macrophages and efferocytosis. Neoplasia 21, 172–184 (2019).
Tang, X. et al. GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Gαq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 73, 6206–6218 (2013).
McGuire, J. J. et al. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat. Commun. 12, 723 (2021).
Coleman, R. E. et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J. Bone Oncol. 13, 123–135 (2018).
Gnant, M. et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 339–351 (2019).
Coleman, R. et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 21, 60–72 (2020).
Gnant, M. Zoledronic acid in breast cancer: latest findings and interpretations. Ther. Adv. Med. Oncol. 3, 293–301 (2011).
Coleman, R. E. et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 102, 1099–1105 (2010).
Tsourdi, E. et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105, 11–17 (2017).
Rachner, T. D. et al. Challenges in preventing bone loss induced by aromatase inhibitors. J. Clin. Endocrinol. Metab. 105, dgaa463 (2020).
Caraglia, M. et al. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr. Relat. Cancer 13, 7–26 (2006).
Mönkkönen, H. et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br. J. Pharmacol. 147, 437–445 (2006).
Clézardin, P. Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone 48, 71–79 (2011).
Dunford, J. E. et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 296, 235–242 (2001).
Diel, I. J. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998).
Gnant, M. et al. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat. Rev. 38, 407–415 (2012).
Di Salvatore, M. et al. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 44, 139–146 (2011).
Rachner, T. D. et al. Regulation of VEGF by mevalonate pathway inhibition in breast cancer. J. Bone Oncol. 2, 110–115 (2013).
Croucher, P. I. et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J. Bone Min. Res. 18, 482–492 (2003).
Giraudo, E. et al. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin. Invest. 114, 623–633 (2004).
van der Pluijm et al. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J. Clin. Invest. 98, 698–705 (1996).
Hiraga, T. et al. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin. Cancer Res. 10, 4559–4567 (2004).
Rogers, T. L. et al. Macrophages as potential targets for zoledronic acid outside the skeleton – evidence from in vitro and in vivo models. Cell Oncol. 36, 505–514 (2013).
Rogers, T. L. et al. Tumour macrophages as potential targets of bisphosphonates. J. Transl Med. 9, 177 (2011).
Melani, C. et al. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 67, 11438–11446 (2007).
Comito, G. et al. Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts. Oncotarget 8, 118–132 (2017).
Junankar, S. et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 5, 35–42 (2015).
Silva-Santos, B. et al. γδT cells in cancer. Nat. Rev. Immunol. 15, 683–691 (2015).
Gruenbacher, G. et al. Mevalonate metabolism in immuno-oncology. Front. Immunol. 8, 1714 (2017).
Gober, H.-J. et al. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197, 163–168 (2003).
Kunzmann, V. et al. Stimulation of γδ-T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96, 384–392 (2000).
Meraviglia, S. et al. In vivo manipulation of V9V2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 161, 290–297 (2010).
Dieli, F. et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67, 7450–7457 (2007).
Boyle, W. J. et al. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Fata, J. E. et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 (2000).
Gonzalez-Suarez, E. et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468, 103–107 (2010).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Hoskin, P. et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 15, 1397–1406 (2014).
Parker, C. C. et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized Alpharadin in Symptomatic Prostate Cancer trial. Eur. Urol. 73, 427–435 (2018).
Buschhaus, J. M. et al. Targeting disseminated estrogen-receptor-positive breast cancer cells in bone marrow. Oncogene 39, 5649–5662 (2020).
Grabinski, N. et al. Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell Signal. 23, 1952–1960 (2011).
Brown, H. K. et al. Parathyroid hormone (PTH) increases skeletal tumour growth and alters tumour distribution in an in vivo model of breast cancer. Int. J. Mol. Sci. 19, 2920 (2018).
Swami, S. et al. Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight 2, e90874 (2017).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
McDonald, M. M. et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 129, 3452–3464 (2017).
Iyer, S. P. et al. A phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 167, 366–375 (2014).
Florio, M. et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 7, 11505 (2016).
Drake, J. M., Danke, J. R. & Henry, M. D. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol. Ther. 9, 607–614 (2010).
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. USA 100, 10954–10959 (2003).
Carducci, M. A. et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110, 1959–1966 (2007).
Moon, H. H. et al. Castration determines the efficacy of ETAR blockade in a mouse model of prostate cancer bone metastasis. Endocrinology 160, 1786–1796 (2019).
Irelli, A. et al. mTOR links tumor immunity and bone metabolism: what are the clinical implications? Int. J. Mol. Sci. 20, 5841 (2019).
Hurvitz, S. A. et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 16, 816–829 (2015).
Győri, D. S. & Mócsai, A. Osteoclast signal transduction during bone metastasis formation. Front. Cell Dev. Biol. 8, 507 (2020).
Boyce, B. & Xing, L. Src inhibitors in the treatment of metastatic bone disease: rationale and clinical data. Clin. Investig. 1, 1695–1706 (2011).
Tulotta, C. et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin. Cancer Res. 25, 2769–2782 (2019).
Lei, W. et al. The IAP antagonist SM-164 eliminates triple-negative breast cancer metastasis to bone and lung in mice. Sci. Rep. 10, 7004 (2020).
Lu, Z. et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature 579, 284–290 (2020).
Haider, M. T. & Taipaleenmäki, H. Targeting the metastatic bone microenvironment by microRNAs. Front. Endocrinol. 9, 202 (2018).
Kuchimaru, T. et al. A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat. Commun. 9, 2981 (2018).
Acknowledgements
All authors acknowledge funding of their original research by the Deutsche Forschungsgemeinschaft Priority Programme SPP 2084 µBONE. In addition, the work of L.C.H. has been supported by grant HO 1875/27-1 and that of A.B. by grant project-A01, FOR2886 TP02 of the Collaborative Research Centre (CRC) 1181, both from Deutsche Forschungsgemeinschaft. K.P. has received funding from the EFPIA and European Union Innovative Medicines Initiative Joint Undertaking for the research project CANCER-ID (grant 115749), the Deutsche Krebshilfe (grant 70112504) and the European Research Council (ERC; Advanced Investigator Grant INJURMET/834974). The authors thank F. Lademann and A. Offermann for assistance with the figures for this article and T. Reiche and A. Strehle for secretarial support.
Review criteria
We performed a PubMed search for full original and review papers in English published up to September 2020, using the key words “breast cancer”, “prostate cancer”, “bone metastases”, “bone cells”, “bone marrow niches”, “circulating tumour cells” and “disseminated tumour cells”. From this initial search, all authors selected the most recent (generally no older than 2014) and most relevant papers for inclusion, but also included seminal studies published since 2001.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to each stage of the preparation of the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
L.C.H. has received honoraria for clinical trials from Alexion, Amgen, Ascendis Pharma, Novartis and Shire, and as a member of advisory boards from Amgen, Kyowa Kirin International, Shire and UCB. M.R. has received honoraria as a member of advisory boards and for lectures from Amgen and Diasorin. F.J. has received unrestricted grants and support for clinical studies from Alexion, Amgen, Lilly and Novartis, and honoraria for lectures and as a member of advisory boards from Alexion, Amgen, Kyowa Kirin International and Lilly. S.P. has received honoraria as a member of advisory boards and for lectures from AstraZeneca, Bristol Myers Squibb, MetaSystems, MSD, Novartis, Roche and Ventana Medical Systems, and research funds from Boehringer Ingelheim, Bristol Myers Squibb, MSD, Roche and Ventana Medical Systems. K.P. has ongoing patent applications related to circulating tumour cells (pending EU patent application no. 18705153.7 (PCT/EP2018/054052)) and has received honoraria from Agena, Illumina, Menarini, Novartis, Roche and Sanofi, and research funding from European Federation of Pharmaceutical Industries and Associations (EFPIA) partners (Angle, Menarini and Servier) of the CANCER-ID programme of the European Union Innovative Medicines Initiative (IMI) Joint Undertaking. A.B. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks R. Coleman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hofbauer, L.C., Bozec, A., Rauner, M. et al. Novel approaches to target the microenvironment of bone metastasis. Nat Rev Clin Oncol 18, 488–505 (2021). https://doi.org/10.1038/s41571-021-00499-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00499-9
This article is cited by
-
The progressive trend of modeling and drug screening systems of breast cancer bone metastasis
Journal of Biological Engineering (2024)
-
Targeting initial tumour–osteoclast spatiotemporal interaction to prevent bone metastasis
Nature Nanotechnology (2024)
-
Inhibition of orthotopic castration-resistant prostate cancer growth and metastasis in mice by JC VLPs carrying a suicide gene driven by the PSA promoter
Cancer Gene Therapy (2024)
-
Circulating tumor cells shielded with extracellular vesicle-derived CD45 evade T cell attack to enable metastasis
Signal Transduction and Targeted Therapy (2024)
-
Clinical applications of circulating tumor cells in patients with solid tumors
Clinical & Experimental Metastasis (2024)