Abstract
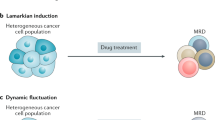
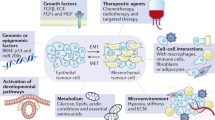
Lineage plasticity, the ability of cells to transition from one committed developmental pathway to another, has been proposed as a source of intratumoural heterogeneity and of tumour adaptation to an adverse tumour microenvironment including exposure to targeted anticancer treatments. Tumour cell conversion into a different histological subtype has been associated with a loss of dependency on the original oncogenic driver, leading to therapeutic resistance. A well-known pathway of lineage plasticity in cancer — the histological transformation of adenocarcinomas to aggressive neuroendocrine derivatives — was initially described in lung cancers harbouring an EGFR mutation, and was subsequently reported in multiple other adenocarcinomas, including prostate cancer in the presence of antiandrogens. Squamous transformation is a subsequently identified and less well-characterized pathway of adenocarcinoma escape from suppressive anticancer therapy. The increased practice of tumour re-biopsy upon disease progression has increased the recognition of these mechanisms of resistance and has improved our understanding of the underlying biology. In this Review, we provide an overview of the impact of lineage plasticity on cancer progression and therapy resistance, with a focus on neuroendocrine transformation in lung and prostate tumours. We discuss the current understanding of the molecular drivers of this phenomenon, emerging management strategies and open questions in the field.
Key points
-
Lineage plasticity can promote both metastasis and therapy resistance.
-
Histological transformation occurs in up to 5% of EGFR-mutant lung adenocarcinomas and at least 20% of prostate adenocarcinomas on targeted therapy.
-
RB1 and p53 deficiency are implicated in — but not sufficient for — neuroendocrine transformation.
-
AKT pathway activation and aberrant activity of the MYC and SOX families of transcriptional regulators have been implicated as being effectors of histological transformation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
17 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41571-020-0355-5
References
Sipos, F., Constantinovits, M. & Muzes, G. Intratumoral functional heterogeneity and chemotherapy. World J. Gastroenterol. 20, 2429–2432 (2014).
Zellmer, V. R. & Zhang, S. Evolving concepts of tumor heterogeneity. Cell Biosci. 4, 69 (2014).
Calbo, J. et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19, 244–256 (2011).
Lim, J. S. et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 545, 360–364 (2017).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Rios, A. C. et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D imaging. Cancer Cell 35, 618–632.e6 (2019).
Hao, Y. et al. TGFbeta signaling limits lineage plasticity in prostate cancer. PLOS Genet. 14, e1007409 (2018).
Guo, W. et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028 (2012).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Grosse-Wilde, A. et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One 10, e0126522 (2015).
McCoy, E. L. et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J. Clin. Invest. 119, 2663–2677 (2009).
Yu, M. et al. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev. 23, 1737–1742 (2009).
Aiello, N. M. et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45, 681–695.e4 (2018).
Reyngold, M. et al. Remodeling of the methylation landscape in breast cancer metastasis. PLoS One 9, e103896 (2014).
Munoz, D. P. et al. Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proc. Natl Acad. Sci. USA 110, E2977–E2986 (2013).
Lopez-Lago, M. A. et al. Genomic deregulation during metastasis of renal cell carcinoma implements a myofibroblast-like program of gene expression. Cancer Res. 70, 9682–9692 (2010).
Carmona, F. J. et al. Epigenetic disruption of cadherin-11 in human cancer metastasis. J. Pathol. 228, 230–240 (2012).
Ezponda, T. et al. The histone methyltransferase MMSET/WHSC1 activates TWIST1 to promote an epithelial-mesenchymal transition and invasive properties of prostate cancer. Oncogene 32, 2882–2890 (2013).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 10, 295–304 (2009).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Maruyama, R. et al. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLOS Genet. 7, e1001369 (2011).
Chaffer, C. L. et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 154, 61–74 (2013).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Chen, S. et al. Conversion of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition is mediated by oxygen concentration in pancreatic cancer cells. Oncol. Lett. 15, 7144–7152 (2018).
Lim, S. et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 31, 512–520 (2010).
Harris, W. J. et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell 21, 473–487 (2012).
Dong, C. et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene 32, 1351–1362 (2013).
Herranz, N. et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell. Biol. 28, 4772–4781 (2008).
Dong, C. et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Invest. 122, 1469–1486 (2012).
Pattabiraman, D. R. et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351, aad3680 (2016).
Wingrove, E. et al. Transcriptomic hallmarks of tumor plasticity and stromal interactions in brain metastasis. Cell Rep. 27, 1277–1292 (2019).
Kiss, M., Van Gassen, S., Movahedi, K., Saeys, Y. & Laoui, D. Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 330, 188–201 (2018).
Park, E. S. et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc. Natl Acad. Sci. USA 108, 17456–17461 (2011).
Sato, R. et al. RNA sequencing analysis reveals interactions between breast cancer or melanoma cells and the tissue microenvironment during brain metastasis. Biomed. Res. Int. 2017, 8032910 (2017).
Tata, P. R. et al. Developmental history provides a roadmap for the emergence of tumor plasticity. Dev. Cell 44, 679–693 (2018).
Zhang, H. et al. Lkb1 inactivation drives lung cancer lineage switching governed by polycomb repressive complex 2. Nat. Commun. 8, 14922 (2017).
Mukhopadhyay, A. et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 8, 40–49 (2014).
Ferone, G. et al. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 30, 519–532 (2016).
Kim, W. et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat. Chem. Biol. 9, 643–650 (2013).
Kwon, O. J., Zhang, L., Ittmann, M. M. & Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl Acad. Sci. USA 111, E592–E600 (2014).
Tata, P. R. & Rajagopal, J. Plasticity in the lung: making and breaking cell identity. Development 144, 755–766 (2017).
Yu, H. A. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 19, 2240–2247 (2013).
Huang, Y., Jiang, X., Liang, X. & Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol. Lett. 15, 6063–6076 (2018).
Zou, M. et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 7, 736–749 (2017).
Bluemn, E. G. et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 32, 474–489 (2017).
Sehrawat, A. et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc. Natl Acad. Sci. USA 115, E4179–E4188 (2018).
Welti, J. et al. Targeting bromodomain and extra-terminal (bet) family proteins in castration-resistant prostate cancer (CRPC). Clin. Cancer Res. 24, 3149–3162 (2018).
Biehs, B. et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 562, 429–433 (2018).
Sanchez-Danes, A. et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 562, 434–438 (2018).
Tsoi, J. et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell 33, 890–904 (2018).
Rambow, F. et al. Toward minimal residual disease-directed therapy in melanoma. Cell 174, 843–855.e19 (2018).
Fallahi-Sichani, M. et al. Adaptive resistance of melanoma cells to RAF inhibition via reversible induction of a slowly dividing de-differentiated state. Mol. Syst. Biol. 13, 905 (2017).
Landsberg, J. et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 490, 412–416 (2012).
Offin, M. et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J. Thorac. Oncol. 14, 1784–1793 (2019).
Lee, J. K. et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 35, 3065–3074 (2017).
Aggarwal, R. et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J. Clin. Oncol. 36, 2492–2503 (2018).
Niederst, M. J. et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 6, 6377 (2015).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Lin, T. et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 7, 165–171 (2005).
Lim, S. T. et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 29, 9–22 (2008).
Gastaldi, S. et al. Met signaling regulates growth, repopulating potential and basal cell-fate commitment of mammary luminal progenitors: implications for basal-like breast cancer. Oncogene 32, 1428–1440 (2013).
Chiche, A. et al. p53 controls the plasticity of mammary luminal progenitor cells downstream of Met signaling. Breast Cancer Res. 21, 13 (2019).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Park, J. W. et al. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 362, 91–95 (2018).
Nakagawa, M., Takizawa, N., Narita, M., Ichisaka, T. & Yamanaka, S. Promotion of direct reprogramming by transformation-deficient Myc. Proc. Natl Acad. Sci. USA 107, 14152–14157 (2010).
Dardenne, E. et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30, 563–577 (2016).
Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 1, 487–495 (2011).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Berger, A. et al. N-Myc-mediated epigenetic reprogramming drives lineage plasticity in advanced prostate cancer. J. Clin. Invest. 130, 3924–3940 (2019).
Chen, Z. et al. Diverse AR-V7 cistromes in castration-resistant prostate cancer are governed by HoxB13. Proc. Natl Acad. Sci. USA 115, 6810–6815 (2018).
Wang, J. et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene 29, 2477–2487 (2010).
Ellwood-Yen, K. et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4, 223–238 (2003).
Kim, J. et al. A mouse model of heterogeneous, c-MYC-initiated prostate cancer with loss of Pten and p53. Oncogene 31, 322–332 (2012).
Farrell, A. S. et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat. Commun. 8, 1728 (2017).
Chen, Y. et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 (2013).
Lee, D. K. et al. Neuroendocrine prostate carcinoma cells originate from the p63-expressing basal cells but not the pre-existing adenocarcinoma cells in mice. Cell Res. 29, 420–422 (2019).
Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
Schaefer, T. & Lengerke, C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: the PI3K/AKT/SOX2 axis and beyond. Oncogene 2, 278–292 (2019).
Rudin, C. M. et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 44, 1111–1116 (2012).
Bass, A. J. et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 41, 1238–1242 (2009).
Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017).
Bhattaram, P. et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat. Commun. 1, 9 (2010).
Sock, E. et al. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol. Cell. Biol. 24, 6635–6644 (2004).
Mosquera, J. M. et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15, 1–10 (2013).
Gong, X. et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 9, 248–263 (2019).
Oser, M. G. et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on Aurora B kinase for survival. Cancer Discov. 9, 230–247 (2019).
Kim, J. et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080 (2017).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 162, 454 (2015).
Blee, A. M. et al. TMPRSS2-ERG controls luminal epithelial lineage and antiandrogen sensitivity in PTEN and TP53-mutated prostate cancer. Clin. Cancer Res. 24, 4551–4565 (2018).
McGranahan, N. et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci. Transl Med. 7, 283ra254 (2015).
Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl Med. 3, 75ra26 (2011).
Beltran, H. et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin. Cancer Res. 22, 1510–1519 (2016).
Roca, E. et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treat. Rev. 59, 117–122 (2017).
Marcoux, N. et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J. Clin. Oncol. 37, 278–285 (2019).
Foster, N. R. et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer 117, 1262–1271 (2011).
Jiang, S. Y. et al. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: a case report and review of literature. Medicine 95, e2752 (2016).
Soo, R. A. et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer 115, 12–20 (2018).
Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 15, 271–286 (2018).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 64, 9209–9216 (2004).
Turbat-Herrera, E. A. et al. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch. Pathol. Lab. Med. 112, 1100–1105 (1988).
Gilani, S., Guo, C. C., Li-Ning, E. M., Pettaway, C. & Troncoso, P. Transformation of prostatic adenocarcinoma to well-differentiated neuroendocrine tumor after hormonal treatment. Hum. Pathol. 64, 186–190 (2017).
Volta, A. D. et al. Transformation of prostate adenocarcinoma into small-cell neuroendocrine cancer under androgen deprivation therapy: much is achieved but more information is needed. J. Clin. Oncol. 37, 350–351 (2019).
Aparicio, A. M. et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 19, 3621–3630 (2013).
Flechon, A. et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann. Oncol. 22, 2476–2481 (2011).
Papandreou, C. N. et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol. 20, 3072–3080 (2002).
Shukuya, T. et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci. 102, 1032–1037 (2011).
Park, S., Han, J. & Sun, J. M. Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 127, 66–68 (2019).
Gong, J. et al. Squamous cell transformation of primary lung adenocarcinoma in a patient with EML4-ALK fusion variant 5 refractory to ALK inhibitors. J. Natl Compr. Canc. Netw. 17, 297–301 (2019).
Scagliotti, G. et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 14, 253–263 (2009).
Izumi, H. et al. Squamous cell carcinoma transformation from EGFR-mutated lung adenocarcinoma: a case report and literature review. Clin. Lung Cancer 19, e63–e66 (2018).
Balanis, N. G. et al. Pan-cancer convergence to a small-cell neuroendocrine phenotype that shares susceptibilities with hematological malignancies. Cancer Cell 36, 17–34 (2019).
Fujita, S., Masago, K., Katakami, N. & Yatabe, Y. Transformation to SCLC after treatment with the ALK inhibitor alectinib. J. Thorac. Oncol. 11, e67–e72 (2016).
Balla, A., Khan, F., Hampel, K. J., Aisner, D. L. & Sidiropoulos, N. Small-cell transformation of ALK-rearranged non-small-cell adenocarcinoma of the lung. Cold Spring Harb. Mol. Case Stud. 4, a002394 (2018).
Le, X. et al. De novo pulmonary small cell carcinomas and large cell neuroendocrine carcinomas harboring EGFR mutations: lack of response to EGFR inhibitors. Lung Cancer 88, 70–73 (2015).
Schartinger, V. H. et al. Neuroendocrine differentiation in head and neck squamous cell carcinoma. J. Laryngol. Otol. 126, 1261–1270 (2012).
Yamagata, K. et al. A case of primary combined squamous cell carcinoma with neuroendocrine (atypical carcinoid) tumor in the floor of the mouth. Case Rep. Dent. 2016, 7532805 (2016).
Mangum, M. D., Greco, F. A., Hainsworth, J. D., Hande, K. R. & Johnson, D. H. Combined small-cell and non-small-cell lung cancer. J. Clin. Oncol. 7, 607–612 (1989).
Rudin, C. M. et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer 19, 289–297 (2019).
Moriguchi, S. et al. Transformation of epidermal growth factor receptor T790M mutation-positive adenosquamous carcinoma of the lung to small cell carcinoma and large-cell neuroendocrine carcinoma following osimertinib therapy: an autopsy case report. Respirol. Case Rep. 7, e00402 (2019).
Priftakis, D., Kritikos, N., Stavrinides, S., Kleanthous, S. & Baziotis, N. Neuroendocrine differentiation in castration-resistant prostate cancer: a case report. Mol. Clin. Oncol. 3, 1392–1394 (2015).
Gluck, G., Mihai, M., Stoica, R., Andrei, R. & Sinescu, I. Prostate cancer with neuroendocrine differentiation — case report. J. Med. Life 5, 101–104 (2012).
Rudin, C. M. et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 18, 3163–3169 (2012).
Puca, L. et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl Med. 11, eaav0891 (2019).
Saunders, L. R. et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl Med. 7, 302ra136 (2015).
Rudin, C. M. et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 18, 42–51 (2017).
Takagi, S. et al. LSD1 inhibitor T-3775440 inhibits SCLC cell proliferation by disrupting LSD1 interactions with SNAG domain proteins INSM1 and GFI1B. Cancer Res. 77, 4652–4662 (2017).
Asangani, I. A. et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 510, 278–282 (2014).
Aggarwal, R. R. et al. Whole-genome and transcriptional analysis of treatment-emergent small-cell neuroendocrine prostate cancer demonstrates intraclass heterogeneity. Mol. Cancer Res. 17, 1235–1240 (2019).
Acknowledgements
The authors’ work is supported by grants from the US National Institutes of Health, including U24CA213274 and R01CA197936 (to C.M.R.).
Author information
Authors and Affiliations
Contributions
A.Q.-V. and J.M.C. researched and drafted the article. H.A.Y., D.P., C.L.S., T.S. and C.M.R. supervised the content. All authors wrote, reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
H.A.Y. has been a consultant on oncology drug development for Astellas Pharma, Astra Zeneca, Daiichi, Lilly, Novartis and Pfizer, and is an inventor on a patent application for pulsatile use of erlotinib to treat or prevent metastases. C.L.S. serves on the board of directors of Novartis, is a co-founder of ORIC Pharmaceuticals and is a co-inventor of enzalutamide and apalutamide. He is a science adviser to Agios, Beigene, Blueprint, Column Group, Foghorn, Housey Pharma, Nextech, KSQ, Petra and PMV. C.M.R. has been a consultant on oncology drug development for AbbVie, Amgen, Ascentage, Astra Zeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Genentech–Roche, Ipsen, Loxo, Pharmamar and Vavotek. He serves on the scientific advisory boards of Bridge Medicines and Harpoon Therapeutics. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks T. Graeber, M. Wicha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quintanal-Villalonga, Á., Chan, J.M., Yu, H.A. et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol 17, 360–371 (2020). https://doi.org/10.1038/s41571-020-0340-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0340-z
This article is cited by
-
Small cells – big issues: biological implications and preclinical advancements in small cell lung cancer
Molecular Cancer (2024)
-
MUC1-C is a target of salinomycin in inducing ferroptosis of cancer stem cells
Cell Death Discovery (2024)
-
FOXA1 and FOXA2: the regulatory mechanisms and therapeutic implications in cancer
Cell Death Discovery (2024)
-
The role of stromal cells in epithelial–mesenchymal plasticity and its therapeutic potential
Discover Oncology (2024)
-
Biophysical control of plasticity and patterning in regeneration and cancer
Cellular and Molecular Life Sciences (2024)