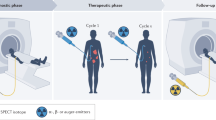
Abstract
Effective patient selection before or early during treatment is important to increasing the therapeutic benefits of anticancer treatments. This selection process is often predicated on biomarkers, predominantly biospecimen biomarkers derived from blood or tumour tissue; however, such biomarkers provide limited information about the true extent of disease or about the characteristics of different, potentially heterogeneous tumours present in an individual patient. Molecular imaging can also produce quantitative outputs; such imaging biomarkers can help to fill these knowledge gaps by providing complementary information on tumour characteristics, including heterogeneity and the microenvironment, as well as on pharmacokinetic parameters, drug–target engagement and responses to treatment. This integrative approach could therefore streamline biomarker and drug development, although a range of issues need to be overcome in order to enable a broader use of molecular imaging in clinical trials. In this Perspective article, we outline the multistage process of developing novel molecular imaging biomarkers. We discuss the challenges that have restricted the use of molecular imaging in clinical oncology research to date and outline future opportunities in this area.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cottingham, M. D., Kalbaugh, C. A. & Fisher, J. A. Tracking the pharmaceutical pipeline: clinical trials and global disease burden. Clin. Transl Sci. 7, 297–299 (2014).
GlobalData Healthcare. Number of pipeline drugs in the US by therapy area. Pharmaceutical Technology https://www.pharmaceutical-technology.com/comment/number-pipeline-drugs-us-therapy-area/ (2017).
Cherny, N. I. et al. ESMO-magnitude of clinical benefit Scale version 1.1. Ann. Oncol. 28, 2340–2366 (2017).
Schnipper, L. E. et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J. Clin. Oncol. 34, 2925–2934 (2016).
Vivot, A. et al. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann. Oncol. 28, 1111–1116 (2017).
Tibau, A. et al. Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration. J. Natl Cancer Inst. 110, 486–492 (2018).
Jaffee, E. M. et al. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 18, e653–e706 (2017).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Menzies, A. M. & Long, G. V. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol. 15, e371–e381 (2014).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Alam, I. S., Arshad, M. A., Nguyen, Q. D. & Aboagye, E. O. Radiopharmaceuticals as probes to characterize tumour tissue. Eur. J. Nucl. Med. Mol. Imag. 42, 537–561 (2015).
O’Connor, J. P. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 14, 169–186 (2017).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer. National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2018).
Lordick, F. et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (Suppl. 5), v50–v57 (2016).
Liu, Y. et al. A risk management approach for imaging biomarker-driven clinical trials in oncology. Lancet Oncol. 16, e622–628 (2015).
Tang, J., Shalabi, A. & Hubbard-Lucey, V. M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 29, 84–91 (2018).
Pharmaceutical Research and Manufacturers of America. Medicines in development for immuno-oncology 2017 report. PhRMA http://www.phrma.org/medicines-in-development-immuno-oncology (2017).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Meignan, M., Gallamini, A. & Haioun, C. Report on the first international workshop on interim-PET scan in lymphoma. Leuk. Lymphoma 50, 1257–1260 (2009).
Barrington, S. F. et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 32, 3048–3058 (2014).
Food and Drug Administration. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. FDA https://www.fda.gov/downloads/drugsGuidanceComplianceRegulatoyInformation/Guidance/UCM071590.pdf (2017).
Schwartz, L. H. et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur. J. Cancer 62, 132–137 (2016).
Janku, F. et al. Outcomes of phase II clinical trials with single-agent therapies in advanced/metastatic non-small cell lung cancer published between 2000 and 2009. Clin. Cancer Res. 18, 6356–6363 (2012).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Litière, S. et al. Validation of RECIST 1.1 for use with cytotoxic agents and targeted cancer agents (TCA): results of a RECIST Working Group analysis of a 50 clinical trials pooled individual patient database [abstract]. J. Clin. Oncol. 35 (Suppl. 15), 2534 (2017).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Seymour, L. et al. RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18, e143–e152 (2017).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50 (Suppl. 1), 122S–150S (2009).
Choi, H. et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 25, 1753–1759 (2007).
Lambin, P. et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48, 441–446 (2012).
Kumar, V. et al. Radiomics: the process and the challenges. Magn. Reson. Imag. 30, 1234–1248 (2012).
Aerts, H. J. W. L. Data science in radiology: a path forward. Clin. Cancer Res. 24, 532–534 (2018).
O’Connor, J. P. et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 21, 249–257 (2015).
Lovinfosse, P. et al. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur. J. Nucl. Med. Mol. Imag. 45, 365–375 (2018).
Li, H. et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 281, 382–391 (2016).
Colen, R. R. et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest. New Drugs 36, 601–607 (2018).
Campbell, M. G. et al. Bridging the gaps in 18F PET tracer development. Nat. Chem. 9, 1–3 (2016).
Decristoforo, C., Penuelas, I., Patt, M. & Todde, S. European regulations for the introduction of novel radiopharmaceuticals in the clinical setting. Q. J. Nucl. Med. Mol. Imag. 61, 135–144 (2017).
Lange, R. et al. Untangling the web of European regulations for the preparation of unlicensed radiopharmaceuticals: a concise overview and practical guidance for a risk-based approach. Nucl. Med. Commun. 36, 412–422 (2015).
van Dongen, G. A., Poot, A. J. & Vugts, D. J. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumor Biol. 33, 607–615 (2012).
Weber, B. et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J. Thorac. Oncol. 6, 1287–1289 (2011).
Saleem, A. et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 5, 30 (2015).
Sun, X. et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci. Transl Med. 10, eaan8840 (2018).
Roesch, F. & Riss, P. J. The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr. Top. Med. Chem. 10, 1633–1668 (2010).
Gebhart, G. et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann. Oncol. 27, 619–624 (2016).
Bensch, F. et al. 89Zr-atezolizumab imaging as non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. https://doi.org/10.1038/s41591-018-0255-8 (2018).
Oosting, S. F. et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 56, 63–69 (2015).
Mestel, R. Cancer: imaging with antibodies. Nature 543, 743–746 (2017).
Gaykema, S. B. et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 20, 3945–3954 (2014).
Sandberg, D. et al. Intra-image referencing for simplified assessment of HER2-expression in breast cancer metastases using the affibody molecule ABY-025 with PET and SPECT. Eur. J. Nucl. Med. Mol. Imag. 44, 1337–1346 (2017).
Orlova, A., Wållberg, H., Stone-Elander, S. & Tolmachev, V. On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J. Nucl. Med. 50, 417–425 (2009).
Malmberg, J., Sandström, M., Wester, K., Tolmachev, V. & Orlova, A. Comparative biodistribution of imaging agents for in vivo molecular profiling of disseminated prostate cancer in mice bearing prostate cancer xenografts: focus on 111In- and 125I-labeled anti-HER2 humanized monoclonal trastuzumab and ABY-025 affibody. Nucl. Med. Biol. 38, 1093–1102 (2011).
Waaijer, S. J. H. et al. Molecular imaging of radiolabeled bispecific T cell engager 89Zr-AMG211 targeting CEA-positive tumors. Clin. Cancer Res. 24, 4988–4996 (2018).
Hanessian, S. Academic-industrial collaboration: toward the consilience of two solitudes. ACS Med. Chem. Lett. 7, 6–9 (2015).
Bernard-Gauthier, V., Collier, T. L., Liang, S. H. & Vasdev, N. Discovery of PET radiopharmaceuticals at the academic-industry interface. Drug Discov. Today Technol. 25, 19–26 (2017).
Saleem, A., Murphy, P., Plisson, C. & Lahn, M. Why are we failing to implement imaging studies with radiolabelled new molecular entities in early oncology drug development? ScientificWorldJournal 2014, 269605 (2014).
European Society of Radiology (ESR). ESR statement on the stepwise development of imaging biomarkers. Insights Imag. 4, 147–152 (2013).
Biomarker, Imaging, and Quality of Life Studies Funding Program. Biomarker study evaluation guidelines. cancer.gov https://www.cancer.gov/about-nci/organization/ccct/funding/biqsfp/2018-biomarker-study-eval-guide.pdf (2018).
Weinreb, J. C. et al. PI-RADS prostate imaging — reporting and data system: 2015, version 2. Eur. Urol. 69, 16–40 (2016).
Sullivan, D. C. et al. Metrology standards for quantitative imaging biomarkers. Radiology 277, 813–825 (2015).
Aide, N. et al. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicenter oncological studies. Eur. J. Nucl. Med. Mol. Imag. 44, 17–25 (2017).
Makris, N. E. et al. Multicenter harmonization of 89Zr PET/CT performance. J. Nucl. Med. 55, 264–267 (2014).
U.S. Food and Drug Administration. CDER biomarker qualification program. FDA https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/default.htm (2018).
McShane, L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Clin. Pract. Oncol. 2, 416–422 (2005).
Caron, J. E., March, J. K., Cohen, M. B. & Schmidt, R. L. A survey of the prevalence and impact of reporting guideline endorsement in pathology journals. Am. J. Clin. Pathol. 148, 314–322 (2017).
Sekula, P., Mallett, S., Altman, D. G. & Sauerbrei, W. Did the reporting of prognostic studies of tumour markers improve since the introduction of REMARK guideline? A comparison of reporting in published articles. PLOS ONE 12, e0178531 (2017).
Strosberg, J. et al. Phase 3 trial of 117Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Kwekkeboom, D. et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 26, 2124–2130 (2008).
Vaupel, P., Mayer, A. & Hockel, M. Tumour hypoxia and malignant progression. Meth. Enzymol. 381, 335–354 (2004).
Lopci, E. et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am. J. Nucl. Med. Mol. Imag. 4, 365–384 (2014).
Welz, S. et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother. Oncol. 124, 526–532 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02352792?term=NCT02352792&rank=1 (2015).
Lee, N. et al. Strategy of using intratreatment hypoxia imaging to selectively and safely guide radiation dose de-escalation concurrent with chemotherapy for locoregionally advanced human papillomavirus related oropharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 96, 9–17 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00606294?term=NCT00606294&rank=1 (2018).
Lamberts, L. E. et al. Antibody positron emission tomography imaging in anticancer drug development. J. Clin. Oncol. 33, 1491–1504 (2015).
Banerji, U. & Workman, P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin. Oncol. 43, 436–445 (2016).
van Kruchten, M. et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 5, 72–81 (2015).
van der Veldt, A. A. et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 21, 82–91 (2012).
Ho, A. L. et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 368, 623–632 (2013).
Rothenberg, S. M., McFadden, D. G., Palmer, E. L., Daniels, G. H. & Wirth, L. J. Redifferentation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 21, 1025–1035 (2015).
Dunn, L. et al. Enhancing radioiodine (RAI) incorporation into BRAFV600E-mutant, RAI-refractory thyroid cancer with the BRAF inhibitor vemurafenib: a pilot study [abstract]. J. Clin. Oncol. 34 (Suppl. 15), 6099 (2016).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Heneweer, C., Holland, J. P., Diviliv, V., Carlin, S. & Lewis, J. S. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J. Nucl. Med. 52, 625–633 (2011).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Nienhuis, H. H. et al. (18)F-fluoroestradiol tumor uptake is heterogeneous and influenced by site of metastasis in breast cancer patients. J. Nucl. Med. 59, 1212–1218 (2018).
Fox, J. J. et al. Positron emission tomography/computed tomography-based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 4, 217–224 (2017).
Ribas, A. & Wolchok, D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Ingram, J. R. et al. PD-L1 is an activation-independent marker of brown adipocytes. Nat. Commun. 8, 647 (2017).
Lesniak, W. G. et al. PD-L1 detection in tumors using (64Cu)atezolizumab with PET. Bioconjug. Chem. 27, 2103–2110 (2016).
Chatterjee, S. et al. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget 7, 10215–10227 (2016).
Hettich, M., Braun, F., Bartholomä, M. D., Schirmbeck, R. & Niedermann, G. Resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics 6, 1629–1640 (2016).
Niemeijer, A. et al. Whole body PD-1 and PD-L1 PET in pts with NSCLC [abstract 1305PD]. Ann. Oncol. 28 (Suppl. 5), v460–v496 (2017).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 (1992).
Sedykh, S. E., Prinz, V. V., Buneva, V. N. & Nevinksky, G. A. Bispecific antibodies: design, therapy, perspectives. Drug Des. Devel. Ther. 12, 195–208 (2018).
Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 17, 197–223 (2017).
Brinkmann, U. & Kontermann, R. E. The making of bispecific antibodies. MAbs 9, 182–212 (2017).
Mandikian, D. et al. Relative target affinities of T cell dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol. Cancer Ther. 17, 776–785 (2018).
Glaudemans, A. W. et al. In vivo and in vitro evidence that 99mTc-HYNIC-interleukin-2 is able to detect T lymphocytes in vulnerable atherosclerotic plaques of the carotid artery. Eur. J. Nucl. Med. Mol. Imag. 41, 1710–1719 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02922283?term=NCT02922283&rank=1 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03107663?term=NCT03107663&rank=1 (2017).
Blykers, A. et al. PET imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18F-radiolabeled Camelid single-domain antibody fragments. J. Nucl. Med. 56, 1265–1271 (2015).
Movahedi, K. et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 72, 4165–4177 (2012).
Jiménez-Sánchez, A. et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170, 927–938 (2017).
Heinzmann, K., Carter, L. M., Lewis, J. S. & Aboagye, E. O. Multiplexed imaging for diagnosis and therapy. Nat. Biomed. Eng. 1, 697–713 (2017).
Lamberts, L. E. et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin. Cancer Res. 23, 2730–2741 (2017).
Koch, M. et al. Threshold analysis and biodistribution of fluorescently labeled bevacizumab in human breast cancer. Cancer Res. 77, 623–631 (2017).
Tunis, S. & Whicher, D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J. Am. Coll. Radiol. 6, 360–365 (2009).
Lindsay, M. J. et al. The National Oncology PET Registry: expanded medicare coverage for PET under coverage with evidence development. Am. J. Roentgenol. 188, 1109–1113 (2007).
Bensch, F. et al. Comparative biodistribution analysis across four different 89Zr-monoclonal antibody tracers – the first step towards an imaging warehouse. Theranostics 8, 4295–4304 (2018).
Beam, A. L. & Kohane, I. S. Big data and machine learning in health care. JAMA 319, 1317–1318 (2018).
Wilhelm-Benartzi, C. S. et al. Challenges and methodology in the incorporation of biomarkers in cancer clinical trials. Crit. Rev. Oncol. Hematol. 110, 49–61 (2017).
Hilgers, R. D., Roes, K. & Stallard, N. Directions for new developments on statistical design and analysis of small population group trials. Orphanet J. Rare Dis. 11, 78 (2016).
Lambin, P. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762 (2017).
IJzerman, M. J., Koffijberg, H., Fenwick, E. & Krahn, M. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics 35, 727–740 (2017).
Doble, B., Tan, M., Harris, A. & Lorgelly, P. Modeling companion diagnostics in economic evaluations of targeted oncology therapies: systemic review and methodological checklist. Expert Rev. Mol. Diagn. 15, 235–254 (2015).
Jong, V. L., Novianti, P. W., Roes, K. C. & Eijkemans, M. J. Selecting a classification function for class prediction with gen expression data. Bioinformatics 32, 1814–1822 (2016).
Bajard, A. et al. An in silico approach helped to identify the best experimental design, population, and outcome for future randomized clinical trials. J. Clin. Epidemiol. 69, 125–136 (2016).
Ventz, S., Alexander, B. M., Parmigiani, G., Gelber, R. D. & Trippa, L. Designing clinical trials that accept new arms: an example in metastatic breast cancer. J. Clin. Oncol. 35, 3160–3168 (2017).
Wallstrom, G., Anderson, K. S. & LaBaer, J. Biomarker discovery for heterogeneous diseases. Cancer Epidemiol. Biomarkers Prev. 22, 747–755 (2013).
Kurland, B. F., Doot, R. K., Linden, H. M., Mankoff, D. A. & Kinahan, P. E. Multicenter trials using 18F-fluorodeoxyglucose (FDG) PET to predict chemotherapy response: effects of differential measurement error and bias on power calculations for unselected and enrichment designs. Clin. Trials 10, 886–995 (2013).
The ABIM Foundation. PET scans after cancer treatment: when you need them — and when you don’t. choosingwisely http://www.choosingwisely.org/patient-resources/pet-scans-after-cancer-treatment/ (2014).
Koleva-Kolarova, R. G. et al. The value of PET/CT with FES or FDG tracers in metastatic breast cancer: a computer simulation study in ER-positive patients. Br. J. Cancer 112, 1617–1625 (2015).
Thorwarth, D. et al. Kinetic analysis of dynamic 18F-fluoromisonidazole PET correlates with radiation treatment outcome in head-and-neck cancer. BMC Cancer 5, 152 (2005).
Rajendran, J. G. et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin. Cancer Res. 12, 5435–5441 (2006).
Rischin, D. et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J. Clin. Oncol. 24, 2098–2104 (2006).
Eschmann, S. M. et al. Hypoxia-imaging with 18F-misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. Radiother. Oncol. 83, 406–410 (2007).
Dirix, P. et al. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with 18F-FDG PET, 18F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J. Nucl. Med. 50, 1020–1027 (2009).
Nehmeh, S. A. et al. Reproducibility of intratumor distribution of 18F fluoromisonidazole in head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 70, 235–242 (2008).
Lee, N. Y. et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 70, 12–13 (2008).
Lee, N. Y. et al. Prospective trial incorporating pre-/mid-treatment [18F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 75, 101–108 (2009).
Kikuchi, M. et al. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann. Nucl. Med. 25, 625–633 (2011).
Yamane, T., Kikuchi, M., Shinohara, S. & Senda, M. Reduction of [18F]fluoromisonidazole uptake after neoadjuvant chemotherapy for head and neck squamous cell carcinoma. Mol. Imag. Biol. 13, 227–231 (2011).
Okamoto, S. et al. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J. Nucl. Med. 54, 201–207 (2013).
Sato, J. et al. 18F-fluoromisonidazole PET uptake is correlated with hypoxia-inducible factor-1α expression in oral squamous cell carcinoma. J. Nucl. Med. 54, 1060–1065 (2013).
Henriques de Figueiredo, B. et al. Potential of [18F]-fluoromisonidazole positron emission tomography for radiotherapy planning in head and neck squamous cell carcinomas. Strahlenther. Onkol. 189, 1015–1019 (2013).
Bittner, M. I. et al. Exploratory geographical analysis of hypoxic subvolumes using 18F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother. Oncol. 108, 511–516 (2013).
Wiedenmann, N. E. et al. Serial [18F]-fluoromisonidazole PET during radiochemotherapy for locally advanced head and neck cancer and its correlation with outcome. Radiother. Oncol. 117, 113–117 (2015).
Okamoto, S. et al. The reoxygenation of hypoxia and the reduction of glucose metabolism in head and neck cancer by fractionated radiotherapy with intensity-modulated radiation therapy. Eur. J. Nucl. Med. Mol. Imag. 43, 2147–2154 (2016).
Grkovski, M. et al. Monitoring early response to chemoradiotherapy with 18F-FMISO dynamic PET in head and neck cancer. Eur. J. Nucl. Med. Mol. Imag. 44, 1682–1691 (2017).
Boeke, S. et al. Geometric analysis of loco-regional recurrences in relation to pre treatment hypoxia in patients with head and neck cancer. Acta Oncol. 56, 1571–1576 (2017).
Zips, D. et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother. Oncol. 105, 21–28 (2012).
Löck, S. et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother. Oncol. 124, 533–540 (2017).
Acknowledgements
The work of the authors is supported by the European Research Council (ERC) Advanced Grant 2011 OnQView (293445), Innovative Medicines Initiative 2 (IMI2) TRISTAN grant 2016 (116106), and Dutch Cancer Society IMPACT (RUG 2012–5565) and POINTING (RUG 2016–10034) grants. The authors would like to thank C. Divgi and A. Glaudemans for their valuable input into this manuscript.
Reviewer information
Nature Reviews Clinical Oncology thanks J. O’Connor, N. Devoogdt, and other anonymous reviewer(s) for their contribution to the peer-review of this work.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
E.G.E.d.V. has served on data safety monitoring boards or advisory boards for Pfizer and Sanofi and has received research funding from Amgen, Chugai, CytomX, Genentech/Roche, Nordic Nanovector, and Regeneron, with all reimbursements and funding made available to her institution (University Medical Center Groningen). The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
American Board of Internal Medicine (ABIM) foundation Choosing Wisely initiative: http://www.choosingwisely.org/
American College of Radiology Accreditation: https://www.acraccreditation.org/
American College of Radiology Imaging Network (ACRIN): https://www.acrin.org/HOME.aspx
ClinicalTrials.gov database: https://clinicaltrials.gov/
EANM Research Ltd (EARL): http://earl.eanm.org/cms/website.php
ESMO-Magnitude of Clinical Benefit Scale: http://www.esmo.org/Policy/Magnitude-of-Clinical-Benefit-Scale
European Association of Nuclear Medicine (EANM): https://www.eanm.org/
European Imaging Biomarkers Alliance: http://www.eibir.org/scientific-activities/joint-initiatives/eiball/
FDA Biomarker Qualification Program: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/default.htm
FDA Clinical Trial Imaging Endpoint Process Standards Guidance for Industry: https://www.fda.gov/downloads/drugs/guidances/ucm268555.pdf
Innovative Medicines Initiative (IMI): http://www.imi.europa.eu/
National Biomarker Development Alliance (NBDA): http://nbdabiomarkers.org
National Biomedical Imaging Archive (NBIA): https://imaging.nci.nih.gov/ncia/login.jsf
National Cancer Institute (NCI) Cancer Imaging Program (CIP): https://imaging.cancer.gov/default.htm
NCI Clinical Trials Working Group (CTWG) Biomarker Study Evaluation Guidelines: https://www.cancer.gov/about-nci/organization/ccct/funding/biqsfp/2017-biomarker-study-eval-guide.pdf
NCI Quantitative Imaging Network (QIN): https://imaging.cancer.gov/programs_resources/specialized_initiatives/qin/about/default.htm
Quantitative Imaging Biomarkers Alliance (QIBA): https://www.rsna.org/QIBA/
Quantitative Imaging Data Warehouse (QIDW): https://www.rsna.org/QIDW/
RECIST criteria: http://recist.eortc.org/
Society of Nuclear Medicine and Molecular Imaging (SNMMI): http://www.snmmi.org/Research/ClinicalTrialsNetwork.aspx?ItemNumber=6831
The Cancer Imaging Archive (TCIA): http://www.cancerimagingarchive.net
Supplementary information
Rights and permissions
About this article
Cite this article
de Vries, E.G.E., Kist de Ruijter, L., Lub-de Hooge, M.N. et al. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat Rev Clin Oncol 16, 241–255 (2019). https://doi.org/10.1038/s41571-018-0123-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0123-y
This article is cited by
-
ImmunoPET imaging of Trop2 in patients with solid tumours
EMBO Molecular Medicine (2024)
-
Evaluation of 18F-AlF-labeled IF7 dimer as a promising molecular probe for tumor targeting PET imaging in mice
Journal of Radioanalytical and Nuclear Chemistry (2024)
-
Current status of contemporary diagnostic radiotracers in the management of breast cancer: first steps toward theranostic applications
EJNMMI Research (2023)
-
Quantification of intratumoural heterogeneity in mice and patients via machine-learning models trained on PET–MRI data
Nature Biomedical Engineering (2023)
-
Design and preclinical evaluation of a novel apelin-based PET radiotracer targeting APJ receptor for molecular imaging of angiogenesis
Angiogenesis (2023)