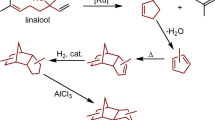
Abstract
Ethanol is presently the most common liquid fuel derived from biomass. One way of meeting the growing demand for heavier middle-distillate fuels — diesel and jet fuels comprising hydrocarbons of typically 8–22 carbon atoms — is to derive these from ethanol. This Review describes the chemistries and processes involved in the conversion of ethanol into diesel and jet fuel drop-in replacements and blendstocks. This conversion of ethanol relies on reactions including dehydration (to olefins), dehydrogenation (to aldehydes), hydrogenation (of C=C and C=O bonds), acid-catalysed olefin oligomerization, metal-catalysed olefin oligomerization, aldolization and ketonization. We discuss the thermodynamics, kinetics, process integration and catalyst development of different approaches. Some routes, particularly those based on olefin oligomerization, have been realized on the pilot scale. Other routes are currently in laboratory stages. This Review provides a framework for understanding how to convert ethanol into distillate-range molecules and the key research problems to be addressed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006).
Alonso, D. M., Bond, J. Q. & Dumesic, J. A. Catalytic conversion of biomass to biofuels. Green Chem. 12, 1493–1513 (2010).
US Department of Energy. Energy policy act of 2005. Energy.gov https://www.energy.gov/downloads/energy-policy-act-2005 (2005).
US Department of Energy. Energy independence and security act of 2007. Congress.gov https://www.congress.gov/bill/110th-congress/house-bill/6 (2007).
US Environmental Protection Agency. Renewable fuel standard program: standards for 2019 and biomass-based diesel volume for 2020. US Federal Register https://www.federalregister.gov/documents/2018/12/11/2018-26566/renewable-fuel-standard-program-standards-for-2019-and-biomass-based-diesel-volume-for-2020 (2018).
Balat, M. & Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energ. 86, 2273–2282 (2009).
US Energy Information Administration. Short-term energy outlook january 2019. EIA.gov https://www.eia.gov/outlooks/steo/ (2019).
Warner, E., Schwab, A. & Bacovsky, D. 2016 survey of non-starch ethanol and renewable hydrocarbon biofuels producers [technical report NREL/TP-6A10-67539]. Energy.gov https://afdc.energy.gov/files/u/publication/2016_survey_non-starch_alcohol_renewable_hydrocarbon_biofuels_producers.pdf (2017).
Silveira, M. H. L. et al. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 8, 3366–3390 (2015).
Lynd, L. R. et al. Cellulosic ethanol: status and innovation. Curr. Opin. Biotechnol. 45, 202–211 (2017).
Wyman, C. E., Cai, C. M. & Kumar, R. Bioethanol from lignocellulosic biomass. Springer https://doi.org/10.1007/978-1-4939-2493-6_521-3 (2017).
Barros, S. Brazil: biofuels annual. USDA.gov https://www.fas.usda.gov/data/brazil-biofuels-annual-4 (2018).
Hsieh, W.-D., Chen, R.-H., Wu, T.-L. & Lin, T.-H. Engine performance and pollutant emission of an SI engine using ethanol–gasoline blended fuels. Atmos. Environ. 36, 403–410 (2002).
Agarwal, A. K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 33, 233–271 (2007).
Hansen, A. C., Zhang, Q. & Lyne, P. W. L. Ethanol–diesel fuel blends — a review. Bioresour. Technol. 96, 277–285 (2005).
Shahir, S. A. et al. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: an assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 32, 379–395 (2014).
ExxonMobil. Outlook for energy: a view to 2040. ExxonMobil https://corporate.exxonmobil.com/en/Energy-and-environment/Energy-resources/Outlook-for-Energy/2018-Outlook-for-Energy-A-View-to-2040 (2017).
Radich, T. The flight paths for biojet fuel. EIA.gov https://www.eia.gov/workingpapers/pdf/flightpaths_biojetffuel.pdf (2015).
World Energy Council. World energy resources: bioenergy 2016. World Energy https://www.worldenergy.org/wp-content/uploads/2017/03/WEResources_Bioenergy_2016.pdf (2016).
Mawhood, R., Gazis, E., de Jong, S., Hoefnagels, R. & Slade, R. Production pathways for renewable jet fuel: a review of commercialization status and future prospects. Biofuel. Bioprod. Biorefin. 10, 462–484 (2016).
Wang, W.-C. et al. Review of biojet fuel conversion technologies [technical report NREL/TP-5100-66291]. NREL.gov https://www.nrel.gov/docs/fy16osti/66291.pdf (2016).
Fellet, M. Now boarding: commercial planes take flight with biobased jet fuel. Chem. Eng. News 94, 16–18 (2016).
Schäfer, A. in Biofuels for Aviation Ch. 1 (ed. Chuck, C.) 3–16 (Academic Press, 2016). This is a detailed book describing the motivations for alternative aviation fuel production, as well as other details on ASTM certification and routes for fuel production.
Bacha, J. et al. Diesel fuels technical review. Chevron Corporation https://www.chevron.com/-/media/chevron/operations/documents/diesel-fuel-tech-review.pdf (2007).
ASTM D1655-17. Standard specification for aviation turbine fuels (ASTM International, 2017).
Hemighaus, G. et al. Aviation Fuels — Technical Review (Chevron Corporation, 2006).
ASTM D7566-18. Standard specification for aviation turbine fuel containing synthesized hydrocarbons (ASTM International, 2018).
De Jong, S. et al. The feasibility of short-term production strategies for renewable jet fuels–a comprehensive techno-economic comparison. Biofuel. Bioprod. Biorefin. 9, 778–800 (2015).
Atsonios, K., Kougioumtzis, M.-A., Panopoulos, K. D. & Kakaras, E. Alternative thermochemical routes for aviation biofuels via alcohols synthesis: process modeling, techno-economic assessment and comparison. Appl. Energ. 138, 346–366 (2015).
Diederichs, G. W., Mandegari, M. A., Farzad, S. & Görgens, J. F. Techno-economic comparison of biojet fuel production from lignocellulose, vegetable oil and sugar cane juice. Bioresour. Technol. 216, 331–339 (2016).
Alves, C. M. et al. Techno-economic assessment of biorefinery technologies for aviation biofuels supply chains in Brazil. Biofuel. Bioprod. Biorefin. 11, 67–91 (2017).
Luning Prak, D. J. et al. Physical and chemical analysis of alcohol-to-jet (ATJ) fuel and development of surrogate fuel mixtures. Energy Fuels 29, 3760–3769 (2015).
ASTM D4054-16. Standard practice for qualification and approval of new aviation turbine fuels and fuel additives (ASTM International, 2016).
Dorrington, G. in Biofuels for Aviation Ch. 3 (ed. Chuck, C.) 35–44 (Academic Press, 2016).
Brooks, K. P. et al. in Biofuels for Aviation Ch. 6 (ed. Chuck, C.) 109–150 (Academic Press, 2016).
ASTM D975-17. Standard specification for diesel fuel oils (ASTM International, 2017).
EN 590:2009. Standard specification on the quality of European diesel fuel (European Standards Organization, 2009).
Yanowitz, J., Ratcliff, M., McCormick, R., Taylor, J. & Murphy, M. Compendium of experimental cetane numbers [technical report NREL/TP-5400-61693]. OSTI.gov https://www.osti.gov/biblio/1150177-compendium-experimental-cetane-numbers (2014).
Santana, R. C. et al. Evaluation of different reaction strategies for the improvement of cetane number in diesel fuels. Fuel 85, 643–656 (2006).
Katritzky, A. R. et al. Quantitative correlation of physical and chemical properties with chemical structure: utility for prediction. Chem. Rev. 110, 5714–5789 (2010).
Saldana, D. A. et al. Flash point and cetane number predictions for fuel compounds using quantitative structure property relationship (QSPR) methods. Energy Fuels 25, 3900–3908 (2011).
Dahmen, M. & Marquardt, W. A novel group contribution method for the prediction of the derived cetane number of oxygenated hydrocarbons. Energy Fuels 29, 5781–5801 (2015).
Kubic Jr, W. L., Jenkins, R. W., Moore, C. M., Semelsberger, T. A. & Sutton, A. D. Artificial neural network based group contribution method for estimating cetane and octane numbers of hydrocarbons and oxygenated organic compounds. Ind. Eng. Chem. Res. 56, 12236–12245 (2017).
Ghosh, P. & Jaffe, S. B. Detailed composition-based model for predicting the cetane number of diesel fuels. Ind. Eng. Chem. Res. 45, 346–351 (2006).
Yinong, L. & Qinghua, D. Investigation of propene oligomerization catalyzed by phosphotungstic acid catalysts. China Pet. Process. Pe. 14, 10–16 (2012).
Jenkins, R. W. et al. The effect of functional groups in bio-derived fuel candidates. ChemSusChem 9, 922–931 (2016).
de Klerk, A. Fischer–Tropsch refining: technology selection to match molecules. Green Chem. 10, (1249–1279 (2008).
Nel, R. J. & de Klerk, A. Dehydration of C5–C12 linear 1-alcohols over η-alumina to fuel ethers. Ind. Eng. Chem. Res. 48, 5230–5238 (2009).
Engelder, C. J. Studies in contact catalysis. J. Phys. Chem. 21, 676–704 (1916).
Adkins, H. & Perkins, P. P. Dehydration of alcohols over alumina. J. Am. Chem. Soc. 47, 1163–1167 (1925).
Fan, D., Dai, D.-J. & Wu, H.-S. Ethylene formation by catalytic dehydration of ethanol with industrial considerations. Materials 6, 101–115 (2013). This is a review of EtOH dehydration to ethylene, with industrial concerns included.
Zhang, M. H. & Yu, Y. Z. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 52, 9505–9514 (2013).
Mohsenzadeh, A., Zamani, A. & Taherzadeh, M. J. Bioethylene production from ethanol: a review and techno-economical evaluation. ChemBioEng Rev. 4, 75–91 (2017).
Yaws, C. L. Chemical Properties Handbook (McGraw-Hill, 1999).
Bailey, B., Eberhardt, J., Goguen, S. & Erwin, J. Diethyl ether (DEE) as a renewable diesel fuel. SAE.org https://doi.org/10.4271/972978 (1997).
Frusteri, F., Spadaro, L., Beatrice, C. & Guido, C. Oxygenated additives production for diesel engine emission improvement. Chem. Eng. J. 134, 239–245 (2007).
Knözinger, H. Dehydration of alcohols on aluminum oxide. Angew. Chem. Int. Ed. 7, 791 (1968).
Roy, S. et al. Mechanistic study of alcohol dehydration on γ-Al2O3. ACS Catal. 2, 1846–1853 (2012).
Kostestkyy, P., Yu, J., Gorte, R. J. & Mpourmpakis, G. Structure–activity relationships on metal-oxides: alcohol dehydration. Catal. Sci. Technol. 4, 3861–3869 (2014).
Kostetskyy, P. & Mpourmpakis, G. Structure–activity relationships in the production of olefins from alcohols and ethers: a first-principles theoretical study. Catal. Sci. Technol. 5, 4547–4555 (2015).
Aronson, M., Gorte, R. & Farneth, W. E. The influence of oxonium ion and carbenium ion stabilities on the alcohol/H-ZSM-5 interaction. J. Catal. 98, 434–443 (1986).
Janik, M. J., Macht, J., Iglesia, E. & Neurock, M. Correlating acid properties and catalytic function: a first-principles analysis of alcohol dehydration pathways on polyoxometalates. J. Phys. Chem. C 113, 1872–1885 (2009).
Chiang, H. & Bhan, A. Catalytic consequences of hydroxyl group location on the rate and mechanism of parallel dehydration reactions of ethanol over acidic zeolites. J. Catal. 271, 251–261 (2010).
Kim, S., Robichaud, D. J., Beckham, G. T., Paton, R. S. & Nimlos, M. R. Ethanol dehydration in HZSM-5 studied by density functional theory: evidence for a concerted peocess. J. Phys. Chem. A 119, 3604–3614 (2015).
Gervasini, A., Fenyvesi, J. & Auroux, A. Study of the acidic character of modified metal oxide surfaces using the test of isopropanol decomposition. Catal. Lett. 43, 219–228 (1997).
Di Cosimo, J., Dıez, V., Xu, M., Iglesia, E. & Apesteguía, C. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 178, 499–510 (1998).
Pines, H. & Haag, W. O. Alumina: catalyst and support. I. Alumina, its intrinsic acidity and catalytic activity. J. Am. Chem. Soc. 82, 2471–2483 (1960).
Knözinger, H. & Köhne, R. Dehydration of alcohols over alumina. 1. Reaction scheme. . J. Catal. 5, 264 (1966).
Chen, G., Li, S., Jiao, F. & Yuan, Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal. Today 125, 111–119 (2007).
Kochar, N. K., Merims, R. & Padia, A. S. Ethylene from ethanol. Chem. Eng. Prog. 77, 66–70 (1981).
El-Katatny, E. A., Halawy, S. A., Mohamed, M. A. & Zaki, M. I. Recovery of ethene-selective FeOx/Al2O3 ethanol dehydration catalyst from industrial chemical wastes. Appl. Catal. A 199, 83–92 (2000).
Tsao, U. & Zasloff, H. B. Production of ethylene from ethanol. US Patent 4134926A (1979).
Le Van Mao, R., Nguyen, T. M. & McLaughlin, G. P. The bioethanol-to-ethylene (B.E.T.E.) process. Appl. Catal. 48, 265–277 (1989).
Phillips, C. B. & Datta, R. Production of ethylene from hydrous ethanol on H-ZSM-5 under mild conditions. Ind. Eng. Chem. Res. 36, 4466–4475 (1997).
Ramesh, K., Jie, C., Han, Y. F. & Borgna, A. Synthesis, characterization, and catalytic activity of phosphorus modified H-ZSM-5 catalysts in selective ethanol dehydration. Ind. Eng. Chem. Res. 49, 4080–4090 (2010).
Takahara, I., Saito, M., Inaba, M. & Murata, K. Dehydration of ethanol into ethylene over solid acid catalysts. Catal. Lett. 105, 249–252 (2005).
Zhang, X., Wang, R. J., Yang, X. X. & Zhang, F. B. Comparison of four catalysts in the catalytic dehydration of ethanol to ethylene. Micropor. Mesopor. Mater. 116, 210–215 (2008).
Varisli, D., Dogu, T. & Dogu, G. Ethylene and diethyl-ether production by dehydration reaction of ethanol over different heteropolyacid catalysts. Chem. Eng. Sci. 62, 5349–5352 (2007).
Saito, Y. & Niiyama, H. Reaction mechanism of ethanol dehydration on/in heteropoly compounds: analysis of transient behavior based on pseudo-liquid catalysis model. J. Catal. 106, 329–336 (1987).
Varisli, D., Dogu, T. & Dogu, G. Silicotungstic acid impregnated MCM-41-like mesoporous solid acid catalysts for dehydration of ethanol. Ind. Eng. Chem. Res. 47, 4071–4076 (2008).
Micek-Ilnicka, A., Bielanska, E., Litynska-Dobrzynska, L. & Bielanski, A. Carbon nanotubes, silica and titania supported heteropolyacid H3PW12O40 as the catalyst for ethanol conversion. Appl. Catal. A 421, 91–98 (2012).
Kozhevnikov, I. Sustainable heterogeneous acid catalysis by heteropoly acids. J. Mol. Catal. A 262, 86–92 (2007).
Huang, H.-J., Ramaswamy, S., Tschirner, U. & Ramarao, B. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 62, 1–21 (2008).
Vane, L. M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuel. Bioprod. Biorefin. 2, 553–588 (2008).
Le Van Mao, R. & Nguyen, T. M. Superacidic catalysts for low temperature conversion of aqueous ethanol to ethylene. US Patent 4847223A (1989).
Nguyen, T. M. & Le Van Mao, R. Conversion of ethanol in aqueous-solution over ZSM-5 zeolites-Study of the reaction network. Appl. Catal. 58, 119–129 (1990).
Chematur Engineering AB. Bio ethylene/ethene. Chematur https://chematur.se/process-areas/bio-chemicals/bio-ethylene-ethene (2019).
Chemicals Technology. Braskem ethanol-to-ethylene plant. Chemicals Technology http://www.chemicals-technology.com/projects/braskem-ethanol/ (2019).
Petron Scientech Inc. Background. Petron Scientech http://www.petronscientech.com/index.php?option=com_content&view=article&id=1&Itemid=115&lang=en (2019).
Ondrey, G. The launch of a new bioethylene-production process. Chem. Engineer. 121, 11–12 (2014).
Technip. Technip completes acquisition of Hummingbird® technology from BP Chemicals Limited. Business Wire https://www.businesswire.com/news/home/20160614006360/en/Technip-Completes-Acquisition-Hummingbird®-Technology-BP-Chemicals (2016).
Lane, J. New path to ethylene via ethanol: the Digest’s 2017 Multi-Slide Guide to Technip’s Hummingbird tech. Biofuels Digest http://www.biofuelsdigest.com/bdigest/2017/01/03/new-path-to-ethylene-via-ethanol-the-digests-2017-multi-slide-guide-to-technips-hummingbird-tech/ (2017).
Ipatieff, V., Corson, B. & Egloff, G. Polymerization, a new source of gasoline. Ind. Eng. Chem. 27, 1077–1081 (1935).
Ipatieff, V. N. & Schaad, R. E. Heptenes and heptanes from propylene and butylenes. Ind. Eng. Chem. 37, 362–364 (1945).
Pines, H. The Chemistry of Catalytic Hydrocarbon Conversions (Elsevier, 2012).
Sarazen, M. L., Doskocil, E. & Iglesia, E. Effects of void environment and acid strength on alkene oligomerization selectivity. ACS Catal. 6, 7059–7070 (2016).
Quann, R. J., Green, L. A., Tabak, S. A. & Krambeck, F. J. Chemistry of olefin oligomerization over ZSM-5 catalyst. Ind. Eng. Chem. Res. 27, 565–570 (1988).
Lukyanov, D. B., Gnep, N. S. & Guisnet, M. R. Kinetic modeling of ethene and propene aromatization over HZSM-5 and GaHZSM-5. Ind. Eng. Chem. Res. 33, 223–234 (1994).
Guisnet, M., Gnep, N. & Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A 89, 1–30 (1992).
Biscardi, J. A. & Iglesia, E. Structure and function of metal cations in light alkane reactions catalyzed by modified H-ZSM5. Catal. Today 31, 207–231 (1996).
Choudhary, V. R., Panjala, D. & Banerjee, S. Aromatization of propene and n-butene over H-galloaluminosilicate (ZSM-5 type) zeolite. Appl. Catal. A 231, 243–251 (2002).
Anderson, J., Mole, T. & Christov, V. Mechanism of some conversions over ZSM-5 catalyst. J. Catal. 61, 477–484 (1980).
Smirniotis, P. G. & Ruckenstein, E. Alkylation of benzene or toluene with MeOH or C2H4 over ZSM-5 or beta zeolite: effect of the zeolite pore openings and of the hydrocarbons involved on the mechanism of alkylation. Ind. Eng. Chem. Res. 34, 1517–1528 (1995).
Degnan, T. F. Jr, Smith, C. M. & Venkat, C. R. Alkylation of aromatics with ethylene and propylene: recent developments in commercial processes. Appl. Catal. A 221, 283–294 (2001).
Hansen, N., Brüggemann, T., Bell, A. T. & Keil, F. J. Theoretical investigation of benzene alkylation with ethene over H-ZSM-5. J. Phys. Chem. C 112, 15402–15411 (2008).
Svelle, S., Kolboe, S. & Swang, O. Theoretical investigation of the dimerization of linear alkenes catalyzed by acidic zeolites. J. Phys. Chem. B 108, 2953–2962 (2004).
Derouane, E. G. et al. Elucidation of the mechanism of conversion of methanol and ethanol to hydrocarbons on a new type of synthetic zeolite. J. Catal. 53, 40–55 (1978). This is an early description of methanol-to-gasoline and EtOH-to-gasoline conversion over H-ZSM-5.
Chang, C. D. & Silvestri, A. J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 47, 249–259 (1977).
Tabak, S. & Yurchak, S. Conversion of methanol over ZSM-5 to fuels and chemicals. Catal. Today 6, 307–327 (1990).
Yurchak, S. Development of Mobil’s fixed-bed methanul-to-gasoline (MTG) process. Stud. Surf. Sci. Catal. 36, 251–272 (1988).
Teketel, S. et al. Shape selectivity in zeolite catalysis. The methanol to hydrocarbons (MTH) reaction. Catalysis 26, 179–217 (2014).
Stöcker, M. Methanol-to-hydrocarbons: catalytic materials and their behavior. Micropor. Mesopor. Mater. 29, 3–48 (1999).
Mole, T., Whiteside, J. A. & Seddon, D. Aromatic co-catalysis of methanol conversion over zeolite catalysts. J. Catal. 82, 261–266 (1983).
Mole, T., Bett, G. & Seddon, D. Conversion of methanol to hydrocarbons over ZSM-5 zeolite: an examination of the role of aromatic hydrocarbons using 13carbon- and deuterium-labeled feeds. J. Catal. 84, 435–445 (1983).
Dahl, I. M. & Kolboe, S. On the reaction mechanism for propene formation in the MTO reaction over SAPO-34. Catal. Lett. 20, 329–336 (1993).
Haw, J. F. et al. Roles for cyclopentenyl cations in the synthesis of hydrocarbons from methanol on zeolite catalyst HZSM-5. J. Am. Chem. Soc. 122, 4763–4775 (2000).
Arstad, B. & Kolboe, S. The reactivity of molecules trapped within the SAPO-34 cavities in the mEtOH-to-hydrocarbons reaction. J. Am. Chem. Soc. 123, 8137–8138 (2001).
Bjørgen, M., Olsbye, U., Petersen, D. & Kolboe, S. The methanol-to-hydrocarbons reaction: insight into the reaction mechanism from [12C]benzene and [13C]methanol coreactions over zeolite H-beta. J. Catal. 221, 1–10 (2004).
Johansson, R., Hruby, S. L., Rass-Hansen, J. & Christensen, C. H. The hydrocarbon pool in ethanol-to-gasoline over HZSM-5 catalysts. Catal. Lett. 127, 1 (2009).
Goguen, P. W. et al. Pulse-quench catalytic reactor studies reveal a carbon-pool mechanism in methanol-to-gasoline chemistry on zeolite HZSM-5. J. Am. Chem. Soc. 120, 2650–2651 (1998).
Xu, T. & White, J. L. Catalyst pretreatment in an oxygenate to olefins reaction system. US Patent US6734330B1 (2004).
Narula, C. K. et al. Heterobimetallic zeolite, InV-ZSM-5, enables efficient conversion of biomass derived ethanol to renewable hydrocarbons. Sci Rep. 5, 16039 (2015).
Talukdar, A. K., Bhattacharyya, K. G. & Sivasanker, S. HZSM-5 catalysed conversion of aqueous ethanol to hydrocarbons. Appl. Catal. A 148, 357–371 (1997).
Gayubo, A. G., Tarrío, A. M., Aguayo, A. T., Olazar, M. & Bilbao, J. Kinetic modelling of the transformation of aqueous ethanol into hydrocarbons on a HZSM-5 zeolite. Ind. Eng. Chem. Res. 40, 3467–3474 (2001).
Aguayo, A. T., Gayubo, A. G., Atutxa, A., Olazar, M. & Bilbao, J. Catalyst deactivation by coke in the transformation of aqueous ethanol into hydrocarbons. Kinetic modeling and acidity deterioration of the catalyst. Ind. Eng. Chem. Res. 41, 4216–4224 (2002).
Madeira, F. F., Gnep, N., Magnoux, P., Maury, S. & Cadran, N. Ethanol transformation over HFAU, HBEA and HMFI zeolites presenting similar Brønsted acidity. Appl. Catal. A 367, 39–46 (2009).
Sun, J. & Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 4, 1078–1090 (2014). This is a general review of the use of EtOH as a platform molecule.
Galadima, A. & Muraza, O. Zeolite catalysts in upgrading of bioethanol to fuels range hydrocarbons: a review. J. Ind. Eng. Chem. 31, 1–14 (2015).
Erichsen, M. W., Svelle, S. & Olsbye, U. The influence of catalyst acid strength on the methanol to hydrocarbons (MTH) reaction. Catal. Today 215, 216–223 (2013).
Costa, E., Uguina, A., Aguado, J. & Hernandez, P. J. Ethanol to gasoline process: effect of variables, mechanism, and kinetics. Ind. Eng. Chem. Process Des. Dev. 24, 239–244 (1985).
Schulz, J. & Bandermann, F. Conversion of ethanol over zeolite H-ZSM-5. Chem. Eng. Technol. 17, 179–186 (1994).
Aguayo, A. T., Gayubo, A. G., Tarrío, A. M., Atutxa, A. & Bilbao, J. Study of operating variables in the transformation of aqueous ethanol into hydrocarbons on an HZSM-5 zeolite. J. Chem. Technol. Biotechnol. 77, 211–216 (2002).
Viswanadham, N., Saxena, S. K., Kumar, J., Sreenivasulu, P. & Nandan, D. Catalytic performance of nano crystalline H-ZSM-5 in ethanol to gasoline (ETG) reaction. Fuel 95, 298–304 (2012).
Chaudhuri, S. N., Halik, C. & Lercher, J. A. Reactions of ethanol over HZSM-5. J. Mol. Catal. 62, 289–295 (1990).
Madeira, F. F. et al. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal. A 443, 171–180 (2012).
Saha, S. K. & Sivasanker, S. Influence of Zn− and Ga-doping on the conversion of ethanol to hydrocarbons over ZSM-5. Catal. Lett. 15, 413–418 (1992).
Inaba, M., Murata, K., Saito, M. & Takahara, I. Ethanol conversion to aromatic hydrocarbons over several zeolite catalysts. React. Kinet. Catal. Lett. 88, 135–141 (2006).
Chang, C., Lang, W. & Smith, R. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts: II. Pressure effects. J. Catal. 56, 169–173 (1979).
Ramasamy, K. K. & Wang, Y. Ethanol conversion to hydrocarbons on HZSM-5: Effect of reaction conditions and Si/Al ratio on the product distributions. Catal. Today 237, 89–99 (2014).
Oudejans, J. C., Van Den Oosterkamp, P. F. & Van Bekkum, H. Conversion of ethanol over zeolite H-ZSM-5 in the presence of water. Appl. Catal. 3, 109–115 (1982).
Hannon, J. One-step high-yield production of fungible gasoline, diesel, and jet fuel blend stocks from ethanol without added hydrogen. Energy.gov https://www.energy.gov/sites/prod/files/2017/05/f34/thermochem_hannon_2.3.1.201.pdf (2017).
El-Halwagi, M. M., Hall, K. R. & Spriggs, H. D. Integrated biofuel processing system. US Patent US8802905B2 (2014).
Warwick, G. Waste watch: airlines and industry pursue biofuels that recycle trash and do no harm to the environment. Air Transport World http://atwonline.com/eco-aviation/waste-watch (2015).
Lane, J. DOE pushes renewable jet fuel towards commercial-scale with key grants to LanzaTech, Byogy, AVAPCO-led teams. Biofuels Digest http://www.biofuelsdigest.com/bdigest/2016/12/30/doe-pushes-renewable-jet-fuel-towards-commercial-scale-with-key-grants-to-lanzatech-byogy-avapco-led-teams/ (2016).
Nelson, K. AVAPCO, BYOGY Renewables, and Petron Scientech announce partnership to demonstrate technologies in the ABBA integrated biorefinery project. PRWeb http://www.prweb.com/releases/2017/10/prweb14773964.htm (2017).
Tian, P., Wei, Y., Ye, M. & Liu, Z. Methanol to olefins (MTO): from fundamentals to commercialization. ACS Catal. 5, 1922–1938 (2015).
Takahashi, A., Xia, W., Nakamura, I., Shimada, H. & Fujitani, T. Effects of added phosphorus on conversion of ethanol to propylene over ZSM-5 catalysts. Appl. Catal. A 423, 162–167 (2012).
Li, X. et al. Light olefins from renewable resources: selective catalytic dehydration of bioethanol to propylene over zeolite and transition metal oxide catalysts. Catal. Today 276, 62–77 (2016).
Ingram, C. W. & Lancashire, R. J. On the formation of C3 hydrocarbons during the conversion of ethanol using H-ZSM-5 catalyst. Catal. Lett. 31, 395–403 (1995).
Lehmann, T. & Seidel-Morgenstern, A. Thermodynamic appraisal of the gas phase conversion of ethylene or ethanol to propylene. Chem. Eng. J. 242, 422–432 (2014).
Lin, B., Zhang, Q. & Wang, Y. Catalytic conversion of ethylene to propylene and butenes over H-ZSM-5. Ind. Eng. Chem. Res. 48, 10788–10795 (2009).
Nicholas, C. P. Applications of light olefin oligomerization to the production of fuels and chemicals. Appl. Catal. A 543, 82–97 (2017). This is a recent review of light olefin oligomerization.
Tabak, S., Krambeck, F. & Garwood, W. Conversion of propylene and butylene over ZSM-5 catalyst. AlChE J. 32, 1526–1531 (1986).
Coelho, A., Caeiro, G., Lemos, M., Lemos, F. & Ribeiro, F. R. 1-Butene oligomerization over ZSM-5 zeolite: part 1 — effect of reaction conditions. Fuel 111, 449–460 (2013).
Martens, J. A., Ravishankar, R., Mishin, I. E. & Jacobs, P. A. Tailored alkene oligomerization with H-ZSM-57 zeolite. Angew. Chem. Int. Ed. 39, 4376–4379 (2000).
Bond, J. Q., Alonso, D. M., Wang, D., West, R. M. & Dumesic, J. A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 327, 1110–1114 (2010).
Kim, Y. T. et al. Low-temperature oligomerization of 1-butene with H-ferrierite. J. Catal. 323, 33–44 (2015).
Nicholas, C. P. in Zeolites in Industrial Separation and Catalysis (ed. Kulprathipanja, S.) 355–402 (Wiley-VCH, 2010).
Martens, L. R., Verduijn, J. & Mathys, G. The development of an environmental friendly catalytic system for the conversion of olefins. Catal. Today 36, 451–460 (1997).
Martens, J. A., Verrelst, W. H., Mathys, G. M., Brown, S. H. & Jacobs, P. A. Tailored catalytic propene trimerization over acidic zeolites with tubular pores. Angew. Chem. Int. Ed. 44, 5687–5690 (2005).
Corma, A., Martínez, C. & Doskocil, E. Designing MFI-based catalysts with improved catalyst life for C= 3 and C= 5 oligomerization to high-quality liquid fuels. J. Catal. 300, 183–196 (2013).
Vaughan, J., Oconnor, C. & Fletcher, J. High-pressure oligomerization of propene over heteropoly acids. J. Catal. 147, 441–454 (1994).
Zhirong, Z., Zaiku, X., Yongfu, C., Refeng, W. & Yaping, Y. Free phosphoric acid of diatomite-phosphate solid acid and its catalytic performance for propylene oligomerization. React. Kinet. Catal. Lett. 70, 379–388 (2000).
Zhang, J., Yan, Y., Chu, Q. & Feng, J. Solid phosphoric acid catalyst for propene oligomerization: effect of silicon phosphate composition. Fuel Process. Technol. 135, 2–5 (2015).
Rylander, P. The Catalytic Hydrogenation in Organic Syntheses 31–63 (Academic Press, 1979).
Bartholomew, C. H. & Farrauto, R. J. in Fundamentals of Industrial Catalytic Processes 411–473 (John Wiley & Sons, 2011).
Davis, R. et al. Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid and enzymatic deconstruction of biomass to sugars and biological conversion of sugars to hydrocarbons [technical report NREL/TP-5100-60223]. NREL.gov https://www.nrel.gov/docs/fy14osti/60223.pdf (2013).
Davis, R. T. et al. Dilute-acid and enzymatic deconstruction of biomass to sugars and catalytic conversion of sugars to hydrocarbons [technical report NREL/TP-5100-62498]. NREL.gov https://www.nrel.gov/docs/fy15osti/62498.pdf (2015).
Dagle, V. L. et al. Integrated process for the catalytic conversion of biomass-derived syngas into transportation fuels. Green Chem. 18, 1880–1891 (2016). This offers a description of the process of converting syngas-derived alcohols into jet fuel through an isobutene intermediate.
Tao, L., Markham, J. N., Haq, Z. & Biddy, M. J. Techno-economic analysis for upgrading the biomass-derived ethanol-to-jet blendstocks. Green Chem. 19, 1082–1101 (2017). This presents a recent techno-economic analysis of the EtOH-to-distillate technology through ethylene oligomerization.
Nicholas, C. P., Rathbun, W. E., Kruse, T. M. & Pham, H. A. Composition of oligomerate. US Patent 9644159B2 (2017).
Nicholas, C. P., Krupa, S. L., Bussche, K. M. V. & Kruse, T. M. Process for making diesel by oligomerization of gasoline. US Patent 9663415B2 (2017).
Catani, R., Mandreoli, M., Rossini, S. & Vaccari, A. Mesoporous catalysts for the synthesis of clean diesel fuels by oligomerisation of olefins. Catal. Today 75, 125–131 (2002).
Knottenbelt, C. Mossgas “gas-to-liquid” diesel fuels—an environmentally friendly option. Catal. Today 71, 437–445 (2002).
Du Toit, F. B. Process and apparatus for the production of diesel fuels by oligomerisation of olefinic feed streams. US Patent 7271304B2 (2007).
de Klerk, A. Distillate production by oligomerization of Fischer−Tropsch olefins over solid phosphoric acid. Energy Fuels 20, 439–445 (2006).
de Klerk, A. Oligomerization of Fischer−Tropsch olefins to distillates over amorphous silica−alumina. Energy Fuels 20, 1799–1805 (2006).
de Klerk, A. Properties of synthetic fuels from H-ZSM-5 oligomerization of Fischer–Tropsch type feed materials. Energy Fuels 21, 3084–3089 (2007).
Avidan, A. Gasoline and distillate fuels from methanol. Stud. Surf. Sci. Catal. 36, 307–323 (1988).
Guillon, E., Cadran, N., Touchais, N. & Bournay, L. Flexible process for transformation of ethanol into middle distillates. US Patent 9475999B2 (2016).
Mazurek, H. Two stage process for catalytic conversion of olefins to higher hydrocarbons. US Patent 4925996 (1990).
Lane, J. The Digest’s 2017 Multi-Slide Guide to LanzaTech/PNNL Syngas-to-ATJ fuels. Biofuels Digest http://www.biofuelsdigest.com/bdigest/2017/08/28/the-digests-2017-multi-slide-guide-to-the-lanzatechpnnl-route-to-fuels-from-biomass-syngasmarch/ (2017).
LanzaTech. LanzaTech awarded $4M from DOE for low carbon jet and diesel demonstration facility. LanzaTech http://www.lanzatech.com/lanzatech-awarded-4m-doe-low-carbon-jet-diesel-demonstration-facility/ (2016).
US Office of Energy Efficiency & Renewable Energy. Energy department announces six projects for pilot- and demonstration-scale manufacturing of biofuels, bioproducts, and biopower. Energy.gov https://www.energy.gov/eere/articles/energy-department-announces-six-projects-pilot-and-demonstration-scale-manufacturing (2016).
ArcelorMittal. ArcelorMittal, LanzaTech and Primetals Technologies announce partnership to construct breakthrough €87m biofuel production facility. ArcelorMittal http://corporate.arcelormittal.com/news-and-media/news/2015/july/13-07-2015 (2015).
LanzaTech. Aemetis acquires license from LanzaTech with California exclusive rights for advanced ethanol from biomass including forest and ag wastes. LanzaTech http://www.lanzatech.com/aemetis-acquires-license-lanzatech-california-exclusive-rights-advanced-ethanol-biomass-including-forest-ag-wastes/ (2016).
ET Energy World. IOC and LanzaTech ink Rs 350 crore pact to construct bio-ethanol facility at Panipat refinery. ET Energy World https://energy.economictimes.indiatimes.com/news/oil-and-gas/ioc-and-lanzatech-to-construct-worlds-first-refinery-off-gas-to-bioethanol-production-facility-at-iocs-panipat-refinery/59529506 (2017).
China News Service. Beijing iron maker to turn waste gas into biofuels. ECNS.cn http://www.ecns.cn/business/2018/02-28/293914.shtml (2018).
Burton, F. Low carbon fuel achieves breakthrough. LanzaTech http://www.lanzatech.com/low-carbon-fuel-project-achieves-breakthrough-lanzatech-produces-jet-fuel-waste-gases-virgin-atlantic/ (2017).
LanzaTech. Virgin Atlantic and LanzaTech celebrate as revolutionary sustainable fuel project takes flight. LanzaTech http://www.lanzatech.com/virgin-atlantic-lanzatech-celebrate-revolutionary-sustainable-fuel-project-takes-flight/#_ftn1 (2018).
McDaniel, M. P. & DesLauriers, P. J. in Kirk-Othmer Encyclopedia of Chemical Technology (Wiley VCH, 2000).
Ziegler, K., Holzkamp, E., Breil, H. & Martin, H. Polymerisation von äthylen und anderen olefinen. Angew. Chem. 67, 426–426 (1955).
Martin, H. & Ziegler, K. Production of dimers and low molecular polymerization products from ethylene. US Patent 2943125 (1960).
Cecchin, G., Morini, G. & Piemontesi, F. in Kirk-Othmer Encyclopedia of Chemical Technology (John Wiley & Sons, Inc., 2000).
Cossee, P. On the reaction mechanism of the ethylene polymerization with heterogeneous Ziegler–Natta catalysts. Tetrahedron Lett. 1, 12–16 (1960).
Cossee, P. Ziegler–Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler–Natta catalysts. J. Catal. 3, 80–88 (1964).
Peuckert, M. & Keim, W. A new nickel complex for the oligomerization of ethylene. Organometallics 2, 594–597 (1983).
Jordan, R. F., Bajgur, C. S., Willett, R. & Scott, B. Ethylene polymerization by a cationic dicyclopentadienyl zirconium(iv) alkyl complex. J. Am. Chem. Soc. 108, 7410–7411 (1986).
Britovsek, G. J. P. et al. Ethylene oligomerization beyond Schulz–Flory distributions. ACS Catal. 5, 6922–6925 (2015).
Forestière, A., Olivier-Bourbigou, H. & Saussine, L. Oligomerization of monoolefins by homogeneous catalysts. Oil Gas Sci. Technol. 64, 649–667 (2009).
Lappin, G. R., Nemec, L. H., Sauer, J. D. & Wagner, J. D. in Kirk-Othmer Encyclopedia of Chemical Technology (John Wiley & Sons, Inc., 2000).
Greiner, E. O. C., Blagoev, M. & Yamaguchi, Y. Chemical Economics Handbook: Linear Alpha-Olefins (IHS Chemical, 2013).
Keim, W. Oligomerization of ethylene to α-olefins: discovery and development of the Shell Higher Olefin Process (SHOP). Angew. Chem. Int. Ed. 52, 12492–12496 (2013).
Skupinska, J. Oligomerization of α-olefins to higher oligomers. Chem. Rev. 91, 613–648 (1991).
Svejda, S. A. & Brookhart, M. Ethylene oligomerization and propylene dimerization using cationic (α-diimine)nickel(ii) catalysts. Organometallics 18, 65–74 (1999).
Gates, D. P. et al. Synthesis of branched polyethylene using (α-diimine) nickel(ii) catalysts: influence of temperature, ethylene pressure, and ligand structure on polymer properties. Macromolecules 33, 2320–2334 (2000).
Zhang, H. et al. Ethylene oligomerization over heterogeneous catalysts. Energy Environ. Focus 3, 246–256 (2014).
Finiels, A., Fajula, F. & Hulea, V. Nickel-based solid catalysts for ethylene oligomerization — a review. Catal. Sci. Technol. 4, 2412–2426 (2014). This presents a review of supported Ni catalysts for ethylene oligomerization.
Brogaard, R. Y. & Olsbye, U. Ethene oligomerization in Ni-containing zeolites: theoretical discrimination of reaction mechanisms. ACS Catal. 6, 1205–1214 (2016).
Joshi, R., Zhang, G., Miller, J. T. & Gounder, R. Evidence for the coordination–insertion mechanism of ethene dimerization at nickel cations exchanged onto beta molecular sieves. ACS Catal. 8, 11407–11422 (2018).
Andrei, R. D., Popa, M. I., Fajula, F. & Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J. Catal. 323, 76–84 (2015).
Toch, K., Thybaut, J., Arribas, M., Martínez, A. & Marin, G. Steering linear 1-alkene, propene or gasoline yields in ethene oligomerization via the interplay between nickel and acid sites. Chem. Eng. Sci. 173, 49–59 (2017).
Lallemand, M., Finiels, A., Fajula, F. & Hulea, V. Catalytic oligomerization of ethylene over Ni-containing dealuminated Y zeolites. Appl. Catal. A 301, 196–201 (2006).
Lallemand, M. et al. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization: Effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A 338, 37–43 (2008).
Martínez, A., Arribas, M. A., Concepción, P. & Moussa, S. New bifunctional Ni–H-beta catalysts for the heterogeneous oligomerization of ethylene. Appl. Catal. A 467, 509–518 (2013).
Moussa, S., Arribas, M. A., Concepción, P. & Martínez, A. Heterogeneous oligomerization of ethylene to liquids on bifunctional Ni-based catalysts: the influence of support properties on nickel speciation and catalytic performance. Catal. Today 277, 78–88 (2016).
Hwang, A. et al. Low temperature oligomerization of ethylene over Ni/Al-KIT catalysts. Catal. Lett. 147, 1303–1314 (2017).
Heveling, J., van der Beek, A. & de Pender, M. Oligomerization of ethene over nickel-exchanged zeolite Y into a diesel-range product. Appl. Catal. 42, 325–336 (1988).
Lacarriere, A. et al. Distillate-range products from non-oil-based sources by catalytic cascade reactions. ChemSusChem 5, 1787–1792 (2012).
Babu, B. H., Lee, M., Hwang, D. W., Kim, Y. & Chae, H.-J. An integrated process for production of jet-fuel range olefins from ethylene using Ni-AlSBA-15 and Amberlyst-35 catalysts. Appl. Catal. A 530, 48–55 (2017).
Schultz, R. G. Olefin dimerization over cobalt-oxide-on-carbon catalysts: III. Oligomerization of ethylene. J. Catal. 7, 286–290 (1967).
Xu, Z. et al. Olefin conversion on nitrogen-doped carbon-supported cobalt catalyst: effect of feedstock. J. Catal. 354, 213–222 (2017).
Emrich, R., Heinemann, O., Jolly, P. W., Krüger, C. & Verhovnik, G. P. J. The role of metallacycles in the chromium-catalyzed trimerization of ethylene. Organometallics 16, 1511–1513 (1997).
Dixon, J. T., Green, M. J., Hess, F. M. & Morgan, D. H. Advances in selective ethylene trimerisation–a critical overview. J. Organomet. Chem. 689, 3641–3668 (2004).
Agapie, T. Selective ethylene oligomerization: recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 255, 861–880 (2011).
Overett, M. J. et al. Mechanistic investigations of the ethylene tetramerisation reaction. J. Am. Chem. Soc. 127, 10723–10730 (2005).
McGuinness, D. S. Olefin oligomerization via metallacycles: dimerization, trimerization, tetramerization, and beyond. Chem. Rev. 111, 2321–2341 (2011).
Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 250, 66–94 (2006).
Berard, S. et al. Process for the production of a fuel base from an ethylene feedstock implementing at least one oligomerization stage in the presence of a homogeneous catalytic system. US Patent US8957270B2 (2015).
Lilga, M. A. et al. Systems and processes for conversion of ethylene feedstocks to hydrocarbon fuels. US Patent 9771533B2 (2017).
Guerbet, M. Action des alcools éthylique, isobutylique, isoamylique, sur leurs dérivés sodés. C. R. Hebd. Acad. Sci. 128, 1002–1004 (1899).
Aitchison, H., Wingad, R. L. & Wass, D. F. Homogeneous ethanol to butanol catalysis — Guerbet renewed. ACS Catal. 6, 7125–7132 (2016).
Galadima, A. & Muraza, O. Catalytic upgrading of bioethanol to fuel grade biobutanol: a review. Ind. Eng. Chem. Res. 54, 7181–7194 (2015).
Frisch, M. J. et al. Gaussian 09, Revision A.02 (Gaussian, Inc., Wallingford, CT, 2016).
Kozlowski, J. T. & Davis, R. J. Heterogeneous catalysts for the Guerbet coupling of alcohols. ACS Catal. 3, 1588–1600 (2013).
Gabriëls, D., Hernández, W. Y., Sels, B., Van Der Voort, P. & Verberckmoes, A. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal. Sci. Technol. 5, 3876–3902 (2015). This presents a thorough review of Guerbet condensation chemistry.
Wu, X. et al. Catalytic upgrading of ethanol to n-butanol: progress in catalyst development. ChemSusChem 11, 71–85 (2018).
Yang, C. & Meng, Z. Bimolecular condensation of ethanol to 1-butanol catalyzed by alkali cation zeolites. J. Catal. 142, 37–44 (1993).
Di Cosimo, J., Apesteguía, C., Ginés, M. & Iglesia, E. Structural requirements and reaction pathways in condensation reactions of alcohols on MgyAlOx catalysts. J. Catal. 190, 261–275 (2000).
Scalbert, J., Thibault-Starzyk, F., Jacquot, R., Morvan, D. & Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 311, 28–32 (2014).
Meunier, F. C., Scalbert, J. & Thibault-Starzyk, F. Unraveling the mechanism of catalytic reactions through combined kinetic and thermodynamic analyses: application to the condensation of ethanol. CR Chim. 18, 345–350 (2015).
Moteki, T. & Flaherty, D. W. Mechanistic insight to C–C bond formation and predictive models for cascade reactions among alcohols on Ca-and Sr-hydroxyapatites. ACS Catal. 6, 4170–4183 (2016).
Ogo, S. et al. 1-Butanol synthesis from ethanol over strontium phosphate hydroxyapatite catalysts with various Sr/P ratios. J. Catal. 296, 24–30 (2012).
Ho, C. R., Shylesh, S. & Bell, A. T. Mechanism and kinetics of ethanol coupling to butanol over hydroxyapatite. ACS Catal. 6, 939–948 (2016).
Tsuchida, T. et al. Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J. Catal. 259, 183–189 (2008).
Ogo, S., Onda, A. & Yanagisawa, K. Selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts. Appl. Catal. A 402, 188–195 (2011).
Hanspal, S., Young, Z. D., Shou, H. & Davis, R. J. Multiproduct steady-state isotopic transient kinetic analysis of the ethanol coupling reaction over hydroxyapatite and magnesia. ACS Catal. 5, 1737–1746 (2015).
Gines, M. J. & Iglesia, E. Bifunctional condensation reactions of alcohols on basic oxides modified by copper and potassium. J. Catal. 176, 155–172 (1998).
Pang, J. et al. Upgrading ethanol to n-butanol over highly dispersed Ni–MgAlO catalysts. J. Catal. 344, 184–193 (2016).
Tu, Y.-J. & Chen, Y.-W. Effects of alkali metal oxide additives on Cu/SiO2 catalyst in the dehydrogenation of ethanol. Ind. Eng. Chem. Res. 40, 5889–5893 (2001).
Ni, M., Leung, D. Y. & Leung, M. K. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 32, 3238–3247 (2007).
Takei, T., Iguchi, N. & Haruta, M. Synthesis of acetoaldehyde, acetic acid, and others by the dehydrogenation and oxidation of ethanol. Catal. Surv. Asia 15, 80–88 (2011).
Hagemeyer, H. J. in Kirk-Othmer Encyclopedia of Chemical Technology (Wiley, 2014).
Wang, C. et al. Low-temperature dehydrogenation of ethanol on atomically dispersed gold supported on ZnZrOx. ACS Catal. 6, 210–218 (2016).
Neurock, M., Tao, Z., Chemburkar, A., Hibbitts, D. D. & Iglesia, E. Theoretical insights into the sites and mechanisms for base catalyzed esterification and aldol condensation reactions over Cu. Faraday Discuss. 197, 59–86 (2017).
Tsuchida, T., Sakuma, S., Takeguchi, T. & Ueda, W. Direct synthesis of n-butanol from ethanol over nonstoichiometric hydroxyapatite. Ind. Eng. Chem. Res. 45, 8634–8642 (2006).
Moteki, T., Rowley, A. T. & Flaherty, D. W. Self-terminated cascade reactions that produce methylbenzaldehydes from ethanol. ACS Catal. 6, 7278–7282 (2016).
Ndou, A. S., Plint, N. & Coville, N. J. Dimerisation of ethanol to butanol over solid-base catalysts. Appl. Catal. A 251, 337–345 (2003).
Ramasamy, K. K. et al. Role of calcination temperature on the hydrotalcite derived MgO–Al2O3 in converting ethanol to butanol. Top. Catal. 59, 46–54 (2016).
Ferrin, P. et al. Modeling ethanol decomposition on transition metals: a combined application of scaling and Brønsted−Evans−Polanyi relations. J. Am. Chem. Soc. 131, (5809–5815 (2009).
Wang, J.-H., Lee, C. & Lin, M. Mechanism of ethanol reforming: theoretical foundations. J. Phys. Chem. C 113, 6681–6688 (2009).
Sun, Z. et al. Efficient catalytic conversion of ethanol to 1-butanol via the Guerbet reaction over copper-and nickel-doped porous. ACS Sustain. Chem. Eng. 5, 1738–1746 (2016).
Marcu, I.-C., Tichit, D., Fajula, F. & Tanchoux, N. Catalytic valorization of bioethanol over Cu-Mg-Al mixed oxide catalysts. Catal. Today 147, 231–238 (2009).
Riittonen, T. et al. Continuous liquid-phase valorization of bio-ethanol towards bio-butanol over metal modified alumina. Renew. Energy 74, 369–378 (2015).
Zhang, C., Borlik, M. & Weiner, H. Coated hydrotalcite catalysts and processes for producing butanol. WO Patent 2014100131A1 (2012).
Zhang, C., Balliet, K. & Johnston, V. J. Catalysts and processes for producing butanol. US Patent 8962897B2 (2015).
Tan, E. C. et al. Comparative techno-economic analysis and process design for indirect liquefaction pathways to distillate-range fuels via biomass-derived oxygenated intermediates upgrading. Biofuel. Bioprod. Biorefin. 11, 41–66 (2017).
Hanspal, S., Young, Z. D., Prillaman, J. T. & Davis, R. J. Influence of surface acid and base sites on the Guerbet coupling of ethanol to butanol over metal phosphate catalysts. J. Catal. 352, 182–190 (2017).
Rao, K. K., Gravelle, M., Valente, J. S. & Figueras, F. Activation of Mg–Al hydrotalcite catalysts for aldol condensation reactions. J. Catal. 173, 115–121 (1998).
Sels, B. F., De Vos, D. E. & Jacobs, P. A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 43, 443–488 (2001).
Gangadharan, A., Shen, M., Sooknoi, T., Resasco, D. E. & Mallinson, R. G. Condensation reactions of propanal over CexZr1−xO2 mixed oxide catalysts. Appl. Catal. A 385, 80–91 (2010).
Shen, W., Tompsett, G. A., Xing, R., Conner, W. C. Jr & Huber, G. W. Vapor phase butanal self-condensation over unsupported and supported alkaline earth metal oxides. J. Catal. 286, 248–259 (2012).
Corma, A. & Iborra, S. Optimization of alkaline earth metal oxide and hydroxide catalysts for base-catalyzed reactions. Adv. Catal. 49, 239–302 (2006).
Jordison, T. L., Lira, C. T. & Miller, D. J. Condensed-phase ethanol conversion to higher alcohols. Ind. Eng. Chem. Res. 54, 10991–11000 (2015).
Tichit, D., Lutic, D., Coq, B., Durand, R. & Teissier, R. The aldol condensation of acetaldehyde and heptanal on hydrotalcite-type catalysts. J. Catal. 219, 167–175 (2003).
Hamilton, C. A., Jackson, S. D. & Kelly, G. J. Solid base catalysts and combined solid base hydrogenation catalysts for the aldol condensation of branched and linear aldehydes. Appl. Catal. A 263, 63–70 (2004).
Norman, D. W., Billodeaux, D. R. & Page, M. D. Dual catalyst system for the self-condensation of alcohols. US Patent 8809594B2 (2014).
Liu, P. & Hensen, E. J. Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 135, 14032–14035 (2013).
Mielby, J. et al. Oxidation of bioethanol using zeolite-encapsulated gold nanoparticles. Angew. Chem. 126, 12721–12724 (2014).
Rekoske, J. E. & Barteau, M. A. Kinetics, selectivity, and deactivation in the aldol condensation of acetaldehyde on anatase titanium dioxide. Ind. Eng. Chem. Res. 50, 41–51 (2010).
Young, Z. D., Hanspal, S. & Davis, R. J. Aldol condensation of acetaldehyde over titania, hydroxyapatite, and magnesia. ACS Catal. 6, 3193–3202 (2016).
Vannice, M. A. & Sen, B. Metal–support effects on the intramolecular selectivity of crotonaldehyde hydrogenation over platinum. J. Catal. 115, 65–78 (1989).
Claus, P. Selective hydrogenation of α, β-unsaturated aldehydes and other C = O and C = C bonds containing compounds. Top. Catal. 5, 51–62 (1998).
Moore, C. M. et al. Acetaldehyde as an ethanol derived bio-building block: an alternative to Guerbet chemistry. Green Chem. 19, 169–174 (2017). This is the first proposal of separating Guerbet chemistries to convert EtOH into heavy aldehydes.
Liang, N., Zhang, X., An, H., Zhao, X. & Wang, Y. Direct synthesis of 2-ethylhexanol via n-butanal aldol condensation–hydrogenation reaction integration over a Ni/Ce-Al2O3 bifunctional catalyst. Green Chem. 17, 2959–2972 (2015).
Nakajima, T., Nameta, H., Mishima, S., Matsuzaki, I. & Tanabe, K. A highly active and highly selective oxide catalyst for the conversion of ethanol to acetone in the presence of water vapour. J. Mater. Chem. 4, 853–858 (1994).
Bussi, J., Parodi, S., Irigaray, B. & Kieffer, R. Catalytic transformation of ethanol into acetone using copper–pyrochlore catalysts. Appl. Catal. A 172, 117–129 (1998).
Nishiguchi, T. et al. Catalytic steam reforming of ethanol to produce hydrogen and acetone. Appl. Catal. A 279, 273–277 (2005).
Murthy, R. S., Patnaik, P., Sidheswaran, P. & Jayamani, M. Conversion of ethanol to acetone over promoted iron oxide catalysis. J. Catal. 109, 298–302 (1988).
Nakajima, T., Tanabe, K., Yamaguchi, T., Matsuzaki, I. & Mishima, S. Conversion of ethanol to acetone over zinc oxide–calcium oxide catalyst: optimization of catalyst preparation and reaction conditions and deduction of reaction mechanism. Appl. Catal. 52, 237–248 (1989).
Rahman, M. M., Davidson, S. D., Sun, J. & Wang, Y. Effect of water on ethanol conversion over ZnO. Top. Catal. 59, 37–45 (2016).
Rodrigues, C. P., Zonetti, P. C., Silva, C. G., Gaspar, A. B. & Appel, L. G. Chemicals from ethanol—The acetone one-pot synthesis. Appl. Catal. A 458, 111–118 (2013).
Orozco, L. M., Renz, M. & Corma, A. Carbon–carbon bond formation and hydrogen production in the ketonization of aldehydes. ChemSusChem 9, 2430–2442 (2016).
Sun, J. et al. Key roles of Lewis acid–base pairs on ZnxZryOz in direct ethanol/acetone to isobutene conversion. J. Am. Chem. Soc. 138, 507–517 (2015).
Iwamoto, M., Mizuno, S. & Tanaka, M. Direct and selective production of propene from bio-ethanol on Sc-loaded In2O3 catalysts. Chem. Eur. J. 19, 7214–7220 (2013).
Sun, J. et al. Direct conversion of bio-ethanol to isobutene on nanosized ZnxZryOz mixed oxides with balanced acid–base sites. J. Am. Chem. Soc. 133, 11096–11099 (2011).
Liu, C., Sun, J., Smith, C. & Wang, Y. A study of ZnxZryOz mixed oxides for direct conversion of ethanol to isobutene. Appl. Catal. A 467, 91–97 (2013).
Saus, A. & Schmidl, E. Benzyl sulfonic acid siloxane as a catalyst: Oligomerization of isobutene. J. Catal. 94, 187–194 (1985).
Hauge, K., Bergene, E., Chen, D., Fredriksen, G. R. & Holmen, A. Oligomerization of isobutene over solid acid catalysts. Catal. Today 100, 463–466 (2005).
Rehfinger, A. & Hoffmann, U. Formation of di-isobutene, main by-product of methyl tertiary butyl ethyl ether synthesis catalyzed by ion exchange resin. Chem. Eng. Technol. 13, 150–156 (1990).
Izquierdo, J., Vila, M., Tejero, J., Cunill, F. & Iborra, M. Kinetic study of isobutene dimerization catalyzed by a macroporous sulphonic acid resin. Appl. Catal. A 106, 155–165 (1993).
Alcántara, R. et al. Trimerization of isobutene over Amberlyst-15 catalyst. React. Funct. Polym. 45, 19–27 (2000).
Yoon, J. W., Chang, J.-S., Lee, H.-D., Kim, T.-J. & Jhung, S. H. Trimerization of isobutene over a zeolite beta catalyst. J. Catal. 245, 253–256 (2007).
Peters, M. W. & Taylor, J. D. Renewable jet fuel blendstock from isobutanol. US Patent 8975461B2 (2015).
Bomgardner, M. M. Gevo isobutyl alcohol power army copter. Chem. Eng. News 92, 11 (2014).
Tsuchida, T., Yoshioka, T., Sakuma, S., Takeguchi, T. & Ueda, W. Synthesis of biogasoline from ethanol over hydroxyapatite catalyst. Ind. Eng. Chem. Res. 47, 1443–1452 (2008).
Lovón-Quintana, J. J., Rodriguez-Guerrero, J. K. & Valença, P. G. Carbonate hydroxyapatite as a catalyst for ethanol conversion to hydrocarbon fuels. Appl. Catal. A 542, 136–145 (2017).
US Environmental Protection Agency. Code of Federal Regulations, pt. 1065, subpart H. Engine fluids, test fuels, analytical gases and other calibration standards. Govinfo.gov https://www.govinfo.gov/content/pkg/CFR-2017-title40-vol37/xml/CFR-2017-title40-vol37-part1065.xml (2017).
Defence Standard 91–091. Turbine fuel, aviation kerosine type, Jet A-1 (UK Defence Standardization, 2016).
US National Library of Medicine. Hazardous substrances data bank. NIH.gov https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm (2019).
Lapuerta, M., Garcia-Contreras, R., Campos-Fernández, J. & Dorado, M. P. Stability, lubricity, viscosity, and cold-flow properties of alcohol−diesel blends. Energy Fuels 24, 4497–4502 (2010).
Acknowledgements
This work was supported by ExxonMobil.
Author information
Authors and Affiliations
Contributions
N.M.E. contributed the majority of the data research, writing and editing of the manuscript. M.D.K. additionally contributed to these areas, as well as to the discussion of content. Substantial discussion of content, reviewing and editing were contributed by J.S.B., J.A.D. and G.W.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Eagan, N.M., Kumbhalkar, M.D., Buchanan, J.S. et al. Chemistries and processes for the conversion of ethanol into middle-distillate fuels. Nat Rev Chem 3, 223–249 (2019). https://doi.org/10.1038/s41570-019-0084-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0084-4
This article is cited by
-
Enhanced ethanol dehydrogenation over Ni-containing zirconia-alumina catalysts with microwave-assisted synthesis
Research on Chemical Intermediates (2024)
-
The synthesis of CdS hierarchical micro-nanostructures with different pore structure and their influence on the photocatalytic ethanol transformation
Research on Chemical Intermediates (2024)
-
Conversion of 1-butanol into High Value-Added Chemicals by Mixed Metal Oxides: The Influence of Co2+, Ni2+, and Zn2+ into Condensed Phase Products Distribution
Catalysis Letters (2024)
-
Enabling direct-growth route for highly efficient ethanol upgrading to long-chain alcohols in aqueous phase
Nature Communications (2023)
-
Acidic graphene organocatalyst for the superior transformation of wastes into high-added-value chemicals
Nature Communications (2023)