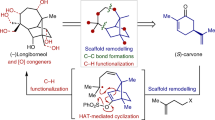
Abstract
Many natural products have intriguing medicinal properties that arise from their fascinating chemical structures. This structural complexity means that the total synthesis of natural products often requires the use of protecting-group chemistry, an approach that is neither economical nor biomimetic. However, structurally complicated and bioactive natural products can be accessible through protecting-group-free (PGF) total syntheses, which are usually much more efficient, provided that the individual reactions proceed with high chemoselectivity. In this Review, we present innovations in methodology and strategy that have enabled the PGF construction of sophisticated organic skeletons bearing multiple asymmetric centres and functional groups. We begin by describing the history of PGF synthesis and then focus on illustrative examples of PGF total syntheses of terpenes and alkaloids reported from 2013 to 2017. These advances will enable more concise and efficient syntheses of molecules of structural and biological importance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Nicolaou, K. C. & Montagnon, T. Molecules That Changed the World. (Wiley-VCH, Weinheim, 2008).
Nicolaou, K. C., Vourloumis, D., Winssinger, N. & Baran, P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 39, 44–122 (2000).
Rodrigues, T., Reker, D., Schneider, P. & Schneider, G. Counting on natural products for drug design. Nat. Chem. 8, 531–541 (2016).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Huang, P.-Q., Yao, Z.-J. & Hsung, R. P. Efficiency in Natural Product Total Synthesis (John Wiley & Sons, 2018).
Hendrickson, J. B. Systematic synthesis design. IV. Numerical codification of construction reactions. J. Am. Chem. Soc. 97, 5784–5800 (1975).
Newhouse, T., Baran, P. S. & Hoffmann, R. W. The economies of synthesis. Chem. Soc. Rev. 38, 3010–3021 (2009).
Burns, N. Z., Baran, P. S. & Hoffmann, R. W. Redox economy in organic synthesis. Angew. Chem. Int. Ed. 48, 2854–2867 (2009).
Trost, B. M. The atom economy — a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Shenvi, R. A., O’Malley, D. P. & Baran, P. S. Chemoselectivity: the mother of invention in total synthesis. Acc. Chem. Res. 42, 530–541 (2009). This is an important account on how total synthesis inspires the invention and innovation of chemoselective chemistry.
Trost, B. M. Selectivity — a key to synthetic efficiency. Science 219, 245–250 (1983). This is a pioneering review of the concept of selectivity regarding the improvement of synthetic efficiency.
Wender, P. A., Verma, V. A., Paxton, T. J. & Pillow, T. H. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41, 40–49 (2008).
Wender, P. A. Toward the ideal synthesis and molecular function through synthesis-informed design. Nat. Prod. Rep. 31, 433–440 (2014).
Wender, P. A., Quiroz, R. V. & Stevens, M. C. Function through synthesis-informed design. Acc. Chem. Res. 48, 752–760 (2015).
Hoffmann, R. W. Protecting-group-free synthesis. Synthesis 21, 3531–3541 (2006). This is the first article reviewing the topic of PGF synthesis.
Young, I. S. & Baran, P. S. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009). This is an excellent review of PGF synthesis with valuable historical insight.
Saicic, R. N. Protecting-group-free syntheses of natural products and biologically active compounds. Tetrahedron 70, 8183–8218 (2014).
Chen, K. & Baran, P. S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature 459, 824–828 (2009).
Shin, I., Wang, G. & Krische, M. J. Catalyst-directed diastereo- and site-selectivity in successive nucleophilic and electrophilic allylations of chiral 1,3-diols: protecting-group-free synthesis of substituted pyrans. Chem. Eur. J. 20, 13382–13389 (2014).
Shin, I., Montgomery, T. P. & Krische, M. J. Catalytic C–C bond formation and the hedricksonian ideal: atom- and redox-economy, stereo- and site-selectivity. Aldrichimica Acta 48, 15 (2015).
Schwartz, L. A. & Krische, M. J. Hydrogen-mediated C–C bond formation: stereo- and site-selective chemical synthesis beyond stoichiometric organometallic reagents. Isr. J. Chem. 58, 45–51 (2018).
Gaich, T. & Baran, P. S. Aiming for the ideal synthesis. J. Org. Chem. 75, 4657–4673 (2010).
Robinson, R. LXIII. — A synthesis of tropinone. J. Chem. Soc. Trans. 111, 762–768 (1917). This article reports that Robinson’s one-step synthesis of tropinone proceeds without protecting groups, at a time when the concept of PGF was underdeveloped.
Willstätter, R. Umwandlung von Tropidin in Tropin. Ber. Dtsch. Chem. Ges. 34, 3163–3165 (1901).
Willstätter, R. III. Synthese des Tropans und Tropidins. Justus Liebigs Ann. Chem. 317, 307–374 (1901).
Willstätter, R. Synthesen in der Tropingruppe. I. Synthese des Tropilidens. Justus Liebigs Ann. Chem. 317, 204–265 (1901).
Willstätter, R. Ueber monocyklische Alkamine der Tropingruppe und eine zweite Synthese des Tropidins. Justus Liebigs Ann. Chem. 326, 1–22 (1903).
Medley, J. W. & Movassaghi, M. Robinson’s landmark synthesis of tropinone. Chem. Commun. 49, 10775–10777 (2013).
Hardegger, E. & Lohse, F. Über Muscarin. 7. Mitteilung. Synthese und absolute Konfiguration des Muscarins. Helv. Chim. Acta 40, 2383–2389 (1957).
Danishefsky, S. J. & Dumas, D. The total synthesis of racemic patchouli and epi-patchouli alcohol. Chem. Commun. 1968, 1287–1288 (1968).
Trost, B. M., Balkovec, J. M. & Mao, M. K. T. A total synthesis of plumericin, allamcin, and allamandin. Part 2. A biomimetic strategy. J. Am. Chem. Soc. 108, 4974–4983 (1986).
Heathcock, C. H., Blumenkopf, T. A. & Smith, K. M. Total synthesis of (±)-fawcettimine. J. Org. Chem. 54, 1548–1562 (1989).
Stoermer, D. & Heathcock, C. H. Total synthesis of (−)-alloaristoteline, (−)-serratoline, and (+)-aristotelone. J. Org. Chem. 58, 564–568 (1993).
Baran, P. S., Maimone, T. J. & Richter, J. M. Total synthesis of marine natural products without using protecting groups. Nature 446, 404–408 (2007). This presents an early and state-of-the-art example of the enantioselective PGF synthesis of structurally complicated alkaloids on a preparative scale.
Muratake, H., Kumagami, H. & Natsume, M. Synthetic studies of marine alkaloids hapalindoles. Part 3. Total synthesis of (±)-hapalindoles H and U. Tetrahedron 46, 6351–6360 (1990).
Baran, P. S. & Richter, J. M. Direct coupling of indoles with carbonyl compounds: short, enantioselective, gram-scale synthetic entry into the hapalindole and fischerindole alkaloid families. J. Am. Chem. Soc. 126, 7450–7451 (2004).
Baran, P. S. & Richter, J. M. Enantioselective total syntheses of welwitindolinone A and fischerindoles I and G. J. Am. Chem. Soc. 127, 15394–15396 (2005).
Pfeiffer, M. W. B. & Phillips, A. J. Total synthesis of (+)-cyanthiwigin U. J. Am. Chem. Soc. 127, 5334–5335 (2005).
Zeng, Y. & Aubé, J. An expeditious total synthesis of (±)-stenine. J. Am. Chem. Soc. 127, 15712–15713 (2005).
McFadden, R. M. & Stoltz, B. M. The catalytic enantioselective, protecting group-free total synthesis of (+)-dichroanone. J. Am. Chem. Soc. 128, 7738–7739 (2006).
Newhouse, T. & Baran, P. S. Total synthesis of (±)-psychotrimine. J. Am. Chem. Soc. 130, 10886–10887 (2008).
Frankowski, K. J., Golden, J. E., Zeng, Y., Lei, Y. & Aubé, J. Syntheses of the Stemona alkaloids (±)-stenine, (±)-neostenine, and (±)-13-epineostenine using a stereodivergent Diels–Alder/azido-Schmidt reaction. J. Am. Chem. Soc. 130, 6018–6024 (2008).
Enquist, J. A. Jr & Stoltz, B. M. The total synthesis of (−)-cyanthiwigin F by means of double catalytic enantioselective alkylation. Nature 453, 1228–1231 (2008). In this paper, two asymmetric quaternary centres are created in one synthetic step (catalytic enantioselective alkylation) in the PGF total synthesis of (−)-cyanthiwigin F.
Hayashida, J. & Rawal, V. H. Total synthesis of ( ± )-platencin. Angew. Chem. Int. Ed. 47, 4373–4376 (2008).
Gaich, T. & Mulzer, J. Total synthesis of (−)-penifulvin A, an insecticide with a dioxafenestrane skeleton. J. Am. Chem. Soc. 131, 452–453 (2009).
Roulland, E. Protecting-group-free total syntheses: a challenging approach. Angew. Chem. Int. Ed. 50, 1226–1227 (2011).
Fernandes, R. A. (ed.). Protecting-Group-Free Organic Synthesis: Improving Economy and Efficiency. (Wiley-VCH, Weinheim, 2018).
Jiménez-Núñez, E., Claverie, C. K., Nieto-Oberhuber, C. & Echavarren, A. M. Prins cyclizations in Au-catalyzed reactions of enynes. Angew. Chem. Int. Ed. 45, 5452–5455 (2006).
Zhou, Q., Chen, X. & Ma, D. Asymmetric, protecting-group-free total synthesis of (−)-englerin A. Angew. Chem. Int. Ed. 49, 3513–3516 (2010).
Willot, M. et al. Total synthesis and absolute configuration of the guaiane sesquiterpene englerin A. Angew. Chem. Int. Ed. 48, 9105–9108 (2009).
Xu, J., Caro-Diaz, E. J. E., Trzoss, L. & Theodorakis, E. A. Nature-inspired total synthesis of (−)-fusarisetin A. J. Am. Chem. Soc. 134, 5072–5075 (2012). In this paper, a nine-step, PGF synthesis of (−)-fusarisetin A through a bioinspired radical cyclization cascade is reported.
Deng, J., Zhu, B., Lu, Z., Yu, H. & Li, A. Total synthesis of (−)-fusarisetin A and reassignment of the absolute configuration of its natural counterpart. J. Am. Chem. Soc. 134, 920–923 (2012).
Corsello, M. A. & Garg, N. K. Synthetic chemistry fuels interdisciplinary approaches to the production of artemisinin. Nat. Prod. Rep. 32, 359–366 (2015).
Zhu, C. & Cook, S. P. A concise synthesis of (+)-artemisinin. J. Am. Chem. Soc. 134, 13577–13579 (2012). This paper discloses a nine-step, gram-scale enantioselective total synthesis of antimalarial (−)-artemisinin from cyclohexenone without protecting-group chemistry.
Schmid, G. & Hofheinz, W. Total synthesis of qinghaosu. J. Am. Chem. Soc. 105, 624–625 (1983).
Qin, H., Xu, Z., Cui, Y. & Jia, Y. Total synthesis of (±)-decursivine and (±)-serotobenine: a Witkop photocyclization/elimination/O-Michael addition cascade approach. Angew. Chem. Int. Ed. 50, 4447–4449 (2011).
Leduc, A. B. & Kerr, M. A. Total synthesis of (±)-decursivine. Eur. J. Org. Chem. 2007, 237–240 (2007).
Yue, G. et al. Collective synthesis of cladiellins based on the gold-catalyzed cascade reaction of 1,7-diynes. Angew. Chem. Int. Ed. 53, 1837–1840 (2014).
Gallou, F. et al. Enantioselective syntheses of authentic sclerophytin A, sclerophytin B, and cladiell-11-ene-3,6,7-triol. Org. Lett. 3, 135–137 (2001).
Zhan, Z.-Y. Recyclable ruthenium catalysts for metathesis reactions. US Patent 20070043180A1 (2007).
Zhan, Z.-Y. Ruthenium complex ligand, ruthenium complex, carried ruthenium complex catalyst and the preparing methods and the use thereof. WO Patent 2007003135A1 (2007).
Wang, B., Ramirez, A. P., Slade, J. J. & Morken, J. P. Enantioselective synthesis of (−)-sclerophytin A by a stereoconvergent epoxide hydrolysis. J. Am. Chem. Soc. 132, 16380–16382 (2010).
Kirillova, M. S., Muratore, M. E., Dorel, R. & Echavarren, A. M. Concise total synthesis of lundurines A–C enabled by gold catalysis and a homodienyl retro-ene/ene isomerization. J. Am. Chem. Soc. 138, 3671–3674 (2016).
Jin, S., Gong, J. & Qin, Y. Total synthesis of (−)-lundurine A and determination of its absolute configuration. Angew. Chem. Int. Ed. 54, 2228–2231 (2015).
Ferrer, C. & Echavarren, A. M. Gold-catalyzed intramolecular reaction of indoles with alkynes: facile formation of eight-membered rings and an unexpected allenylation. Angew. Chem. Int. Ed. 45, 1105–1109 (2006).
Frank, É. et al. Efficient approach to androstene-fused arylpyrazolines as potent antiproliferative agents. Experimental and theoretical studies of substituent effects on BF3-catalyzed intramolecular [3+2] cycloadditions of olefinic phenylhydrazones. J. Am. Chem. Soc. 131, 3894–3904 (2009).
Hudlicky, T. & Koszyk, F. J. Selectivity in retro-ene versus cyclopentene rearrangements of a cis-methylvinylcyclopropane. Tetrahedron Lett. 21, 2487–2490 (1980).
Newcomb, E. T., Knutson, P. C., Pedersen, B. A. & Ferreira, E. M. Total synthesis of gelsenicine via a catalyzed cycloisomerization strategy. J. Am. Chem. Soc. 138, 108–111 (2016).
Harada, T., Shimokawa, J. & Fukuyama, T. Unified total synthesis of five gelsedine-type alkaloids: (−)-gelsenicine, (−)-gelsedine, (−)-gelsedilam, (−)-14-hydroxygelsenicine, and (−)-14,15-dihydroxygelsenicine. Org. Lett. 18, 4622–4625 (2016).
Nieto-Oberhuber, C. et al. Gold(I)-catalyzed intramolecular cyclopropanation of dienynes. Chem. Eur. J. 12, 1694–1702 (2006).
Li, H., Cheng, P., Jiang, L., Yang, J.-L. & Zu, L. Bio-inspired fragmentations: rapid assembly of indolones, 2-quinolinones, and (−)-goniomitine. Angew. Chem. Int. Ed. 56, 2754–2757 (2017).
Takano, S., Sato, T., Inomata, K. & Ogasawara, K. The enantiocontrolled total synthesis of natural (−)-goniomitine. J. Chem. Soc. Chem. Commun. 462–464 (1991).
Pritchett, B. P., Kikuchi, J., Numajiri, Y. & Stoltz, B. M. Enantioselective Pd-catalyzed allylic alkylation reactions of dihydropyrido[1,2-a]indolone substrates: efficient syntheses of (−)-goniomitine, (+)-aspidospermidine, and (−)-quebrachamine. Angew. Chem. Int. Ed. 55, 13529–13532 (2016).
Schmiedel, V. M., Hong, Y. J., Lentz, D., Tantillo, D. J. & Christmann, M. Synthesis and structure revision of dichrocephones A and B. Angew. Chem. Int. Ed. 57, 2419–2422 (2018).
Werner, T., Hoffmann, M. & Deshmukh, S. First enantioselective catalytic Wittig reaction. Eur. J. Org. Chem. 2014, 6630–6633 (2014).
Kotha, S. & Aswar, V. R. Target specific tactics in olefin metathesis: synthetic approach to cis-syn-cis-triquinanes and -propellanes. Org. Lett. 18, 1808–1811 (2016).
Isayama, S. & Mukaiyama, T. A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(ii). Chem. Lett. 18, 1071–1074 (1989).
Daub, M. E., Prudhomme, J., Le Roch, K. & Vanderwal, C. D. Synthesis and potent antimalarial activity of kalihinol B. J. Am. Chem. Soc. 137, 4912–4915 (2015).
Chi, Y. & Gellman, S. H. Diphenylprolinol methyl ether: a highly enantioselective catalyst for Michael addition of aldehydes to simple enones. Org. Lett. 7, 4253–4256 (2005).
McGarraugh, P. G. & Brenner-Moyer, S. E. An organocascade kinetic resolution. Org. Lett. 13, 6460–6463 (2011).
Pronin, S. V., Reiher, C. A. & Shenvi, R. A. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature 501, 195–199 (2013).
Pronin, S. V., Reiher, C. A. & Shenvi, R. A. Corrigendum: stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature 503, 300 (2013).
Feng, J., Noack, F. & Krische, M. J. Modular terpenoid construction via catalytic enantioselective formation of all-carbon quaternary centers: total synthesis of oridamycin A, triptoquinones B and C, and isoiresin. J. Am. Chem. Soc. 138, 12364–12367 (2016).
Meng, Z. et al. Total synthesis and antiviral activity of indolosesquiterpenoids from the xiamycin and oridamycin families. Nat. Commun. 6, 6096 (2015).
Feng, J., Garza, V. J. & Krische, M. J. Redox-triggered C–C coupling of alcohols and vinyl epoxides: diastereo- and enantioselective formation of all-carbon quaternary centers via tert-(hydroxy)-prenylation. J. Am. Chem. Soc. 136, 8911–8914 (2014).
Strom, A. E. & Hartwig, J. F. One-pot anti-Markovnikov hydroamination of unactivated alkenes by hydrozirconation and amination. J. Org. Chem. 78, 8909–8914 (2013).
Liu, Y.-T., Li, L.-P., Xie, J.-H. & Zhou, Q.-L. Divergent asymmetric total synthesis of mulinane diterpenoids. Angew. Chem. Int. Ed. 56, 12708–12711 (2017).
Liu, Y.-T. et al. Asymmetric hydrogenation of tetrasubstituted cyclic enones to chiral cycloalkanols with three contiguous stereocenters. Org. Lett. 19, 3231–3234 (2017).
Adam, W. et al. Synthesis of the endoperoxide anti-7,8-dioxatricyclo[4.2.2.02,5]deca-3,9-diene via singlet oxygenation of the bicyclic valence tautomer of cyclooctatetraene and its transformations. J. Am. Chem. Soc. 103, 5822–5828 (1981).
Suzuki, M., Ohtake, H., Kameya, Y., Hamanaka, N. & Noyori, R. Ruthenium(ii)-catalyzed reactions of 1,4-epiperoxides. J. Org. Chem. 54, 5292–5302 (1989).
Xie, J.-H. & Zhou, Q.-L. Chiral diphosphine and monodentate phosphorus ligands on a spiro scaffold for transition-metal-catalyzed asymmetric reactions. Acc. Chem. Res. 41, 581–593 (2008).
Hernandez, L. W., Pospech, J., Klöckner, U., Bingham, T. W. & Sarlah, D. Synthesis of (+)-pancratistatins via catalytic desymmetrization of benzene. J. Am. Chem. Soc. 139, 15656–15659 (2017).
Tian, X., Hudlicky, T. & Königsberger, K. First total synthesis of (+)-pancratistatin: an unusual set of problems. J. Am. Chem. Soc. 117, 3643–3644 (1995).
Hernandez, L. W., Klöckner, U., Pospech, J., Hauss, L. & Sarlah, D. Nickel-catalyzed dearomative trans-1,2-carboamination. J. Am. Chem. Soc. 140, 4503–4507 (2018).
Berkessel, A. & Adrio, J. A. Dramatic acceleration of olefin epoxidation in fluorinated alcohols: activation of hydrogen peroxide by multiple H-bond networks. J. Am. Chem. Soc. 128, 13412–13420 (2006).
Byers, J. A. & Jamison, T. F. Entropic factors provide unusual reactivity and selectivity in epoxide-opening reactions promoted by water. Proc. Natl Acad. Sci. USA 110, 16724–16729 (2013).
Brunet, J. J., Sidot, C. & Caubere, P. Sunlamp-irradiated phase-transfer catalysis. 1. Cobalt carbonyl catalyzed SRN1 carbonylations of aryl and vinyl halides. J. Org. Chem. 48, 1166–1171 (1983).
Karimi, B. & Golshani, B. Mild and highly efficient method for the silylation of alcohols using hexamethyldisilazane catalyzed by iodine under nearly neutral reaction conditions. J. Org. Chem. 65, 7228–7230 (2000).
Tezuka, N. et al. Direct hydroxylation and amination of arenes via deprotonative cupration. J. Am. Chem. Soc. 138, 9166–9171 (2016).
Liu, Y., Virgil, S. C., Grubbs, R. H. & Stoltz, B. M. Palladium-catalyzed decarbonylative dehydration for the synthesis of α-vinyl carbonyl compounds and total synthesis of (−)-aspewentins A, B, and C. Angew. Chem. Int. Ed. 54, 11800–11803 (2015).
Liu, Y. et al. Palladium-catalyzed decarbonylative dehydration of fatty acids for the production of linear alpha olefins. Adv. Synth. Catal. 356, 130–136 (2014).
Mohr, J. T., Behenna, D. C., Harned, A. M. & Stoltz, B. M. Deracemization of quaternary stereocenters by Pd-catalyzed enantioconvergent decarboxylative allylation of racemic β-ketoesters. Angew. Chem. Int. Ed. 44, 6924–6927 (2005).
Marziale, A. N. et al. An efficient protocol for the palladium-catalyzed asymmetric decarboxylative allylic alkylation using low palladium concentrations and a palladium(ii) precatalyst. Adv. Synth. Catal. 357, 2238–2245 (2015).
Hu, X. & Maimone, T. J. Four-step synthesis of the antimalarial cardamom peroxide via an oxygen stitching strategy. J. Am. Chem. Soc. 136, 5287–5290 (2014).
Kornblum, N. & DeLaMare, H. E. The base catalyzed decomposition of a dialkyl peroxide. J. Am. Chem. Soc. 73, 880–881 (1951).
Maimone, T. J. & Baran, P. S. Modern synthetic efforts toward biologically active terpenes. Nat. Chem. Biol. 3, 396–407 (2007).
Beatty, J. W. & Stephenson, C. R. J. Synthesis of (−)-pseudotabersonine, (−)-pseudovincadifformine, and (+)-coronaridine enabled by photoredox catalysis in flow. J. Am. Chem. Soc. 136, 10270–10273 (2014).
Lowry, M. S. et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(iii) complex. Chem. Mater. 17, 5712–5719 (2005).
Freeman, D. B., Furst, L., Condie, A. G. & Stephenson, C. R. J. Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light. Org. Lett. 14, 94–97 (2012).
Kuehne, M. E., Kirkemo, C. L., Matsko, T. H. & Bohnert, J. C. Studies in biomimetic alkaloid syntheses. 5. Studies syntheses of ψ-vincadifformine, 20-epi-ψ-vincadifformine, pandoline, 20-epipandoline, and the C-16 epimeric(carbomethoxy)velbanamines. J. Org. Chem. 45, 3259–3265 (1980).
Xu, J. et al. Construction of tetracyclic 3-spirooxindole through cross-dehydrogenation of pyridinium: applications in facile synthesis of (±)-corynoxine and (±)-corynoxine B. J. Am. Chem. Soc. 136, 17962–17965 (2014).
Takayama, H. et al. The first total synthesis of (–)-mitragynine, an analgesic indole alkaloid in Mitragyna speciosa. Tetrahedron Lett. 36, 9337–9340 (1995).
Wanner, M. J., Ingemann, S., van Maarseveen, J. H. & Hiemstra, H. Total synthesis of the spirocyclic oxindole alkaloids corynoxine, corynoxine B, corynoxeine, and rhynchophylline. Eur. J. Org. Chem. 2013, 1100–1106 (2013).
Ebner, C. & Carreira, E. M. Pentafulvene for the synthesis of complex natural products: total syntheses of (±)-pallambins A and B. Angew. Chem. Int. Ed. 54, 11227–11230 (2015).
Denmark, S. E. & Edwards, J. P. A. Comparison of (chloromethyl)- and (iodomethyl)zinc cyclopropanation reagents. J. Org. Chem. 56, 6974–6981 (1991).
Li, Z. et al. Total synthesis of crisamicin A. Org. Lett. 10, 3017–3020 (2008).
Liu, W. et al. Scalable total synthesis of rac-jungermannenones B and C. Angew. Chem. Int. Ed. 55, 3112–3116 (2016).
Youn, S. W., Pastine, S. J. & Sames, D. Ru(iii)-catalyzed cyclization of arene-alkene substrates via intramolecular electrophilic hydroarylation. Org. Lett. 6, 581–584 (2004).
Martinez, L. P., Umemiya, S., Wengryniuk, S. E. & Baran, P. S. 11-Step total synthesis of pallambins C and D. J. Am. Chem. Soc. 138, 7536–7539 (2016).
Xu, X.-S., Li, Z.-W., Zhang, Y.-J., Peng, X.-S. & Wong, H. N. C. Total synthesis of (±)-pallambins C and D. Chem. Commun. 48, 8517–8519 (2012).
Finkbeiner, P., Murai, K., Röpke, M. & Sarpong, R. Total synthesis of terpenoids employing a “benzannulation of carvone” strategy: synthesis of (–)-crotogoudin. J. Am. Chem. Soc. 139, 11349–11352 (2017).
Breitler, S. & Carreira, E. M. Total synthesis of ( + )-crotogoudin. Angew. Chem. Int. Ed. 52, 11168–11171 (2013).
Murali, D. & Rao, G. S. K. Benzocyclization of 2,4-hexadienoic acids. Synthesis of (R)-(−)-curcuphenol acetate. Synthesis 1987, 254–256 (1987).
Yu, X. et al. Enantioselective total syntheses of various amphilectane and serrulatane diterpenoids via Cope rearrangements. J. Am. Chem. Soc. 138, 6261–6270 (2016).
Liu, L.-Z., Han, J.-C., Yue, G.-Z., Li, C.-C. & Yang, Z. Asymmetric total synthesis of caribenol A. J. Am. Chem. Soc. 132, 13608–13609 (2010).
Han, J.-C., Liu, L.-Z., Li, C.-C. & Yang, Z. Asymmetric, protecting-group-free total synthesis of ( + )-caribenol A. Chem. Asian J. 8, 1972–1975 (2013).
Hao, H.-D. & Trauner, D. Furans as versatile synthons: total syntheses of caribenol A and caribenol B. J. Am. Chem. Soc. 139, 4117–4122 (2017).
Zhao, X.-H. et al. Total synthesis of (±)-lycojaponicumin D and lycodoline-type lycopodium alkaloids. J. Am. Chem. Soc. 139, 7095–7103 (2017).
Piemontesi, C., Wang, Q. & Zhu, J. Enantioselective total synthesis of (−)-terengganensine A. Angew. Chem. Int. Ed. 55, 6556–6560 (2016).
Evanno, L., Ormala, J. & Pihko, P. M. A highly enantioselective access to tetrahydroisoquinoline and β-carboline alkaloids with simple Noyori-type catalysts in aqueous media. Chem. Eur. J. 15, 12963–12967 (2009).
Huang, B., Guo, L. & Jia, Y. Protecting-group-free enantioselective synthesis of (−)-pallavicinin and (+)-neopallavicinin. Angew. Chem. Int. Ed. 54, 13599–13603 (2015).
Peng, X.-S. & Wong, H. N. C. Total synthesis of (±)-pallavicinin and (±)-neopallavicinin. Chem. Asian J. 1, 111–120 (2006).
Mizoguchi, H., Oikawa, H. & Oguri, H. Biogenetically inspired synthesis and skeletal diversification of indole alkaloids. Nat. Chem. 6, 57–64 (2014).
Szántay, C., Bölcskei, H. & Gács-Baitz, E. Synthesis of vinca alkaloids and related-compounds XLVIII synthesis of (+)-catharanthine and (±)-allocatharanthine. Tetrahedron 46, 1711–1732 (1990).
Zhao, Y.-M. & Maimone, T. J. Short, enantioselective total synthesis of chatancin. Angew. Chem. Int. Ed. 54, 1223–1226 (2015).
Soucy, P., L’Heureux, A., Toró, A. & Deslongchamps, P. Pyranophane transannular Diels–Alder approach to (+)-chatancin: a biomimetic asymmetric total synthesis. J. Org. Chem. 68, 9983–9987 (2003).
Kondoh, A., Arlt, A., Gabor, B. & Fürstner, A. Total synthesis of nominal gobienine A. Chem. Eur. J. 19, 7731–7738 (2013).
Lam, H. C., Pepper, H. P., Sumby, C. J. & George, J. H. Biomimetic total synthesis of (±)-verrubenzospirolactone. Angew. Chem. Int. Ed. 56, 8532–8535 (2017).
Yang, P., Yao, M., Li, J., Li, Y. & Li, A. Total synthesis of rubriflordilactone B. Angew. Chem. Int. Ed. 55, 6964–6968 (2016).
Yu, J., Gaunt, M. J. & Spencer, J. B. Convenient preparation of trans-arylalkenes via palladium(ii)-catalyzed isomerization of cis-arylalkenes. J. Org. Chem. 67, 4627–4629 (2002).
Nannini, L. J., Nemat, S. J. & Carreira, E. M. Total synthesis of (+)-sarcophytin. Angew. Chem. Int. Ed. 57, 823–826 (2018).
Corey, E. J. & Myers, A. G. Total synthesis of (±)-antheridium-inducing factor (AAn) of the fern Anemia Phyllitidis. Clarification of stereochemistry. J. Am. Chem. Soc. 107, 5574–5576 (1985).
Krüger, S. & Gaich, T. Enantioselective, protecting-group-free total synthesis of sarpagine alkaloids — a generalized approach. Angew. Chem. Int. Ed. 54, 315–317 (2015).
Wang, T. & Cook, J. M. General approach for the synthesis of sarpagine/ajmaline indole alkaloids. Stereospecific total synthesis of the sarpagine alkaloid (+)-vellosimine. Org. Lett. 2, 2057–2059 (2000).
Aggarwal, V. K. et al. (1 R,3 R)-2-Methylene-1,3-dithiolane 1,3-dioxide: a highly reactive and selective chiral ketene equivalent. J. Org. Chem. 60, 4962–4963 (1995).
Chen, B. et al. Enantioselective total synthesis of (−)-colchicine, (+)-demecolcinone and metacolchicine: determination of the absolute configurations of the latter two alkaloids. Chem. Sci. 8, 4961–4966 (2017).
Achmatowicz, O., Bukowski, P., Szechner, B., Zwierzchowska, Z. & Zamojski, A. Synthesis of methyl 2,3-dideoxy-dl-alk-2-enopyranosides from furan compounds: a general approach to the total synthesis of monosaccharides. Tetrahedron 27, 1973–1996 (1971).
Lee, J. C., Jin, S.-j & Cha, J. K. Total synthesis of colchicine. α-Methoxy-substituted oxyallyl [4+3] cycloaddition approach. J. Org. Chem. 63, 2804–2805 (1998).
Banwell, M. G. Cyclopropyl compounds as chemical building blocks: total syntheses of the alkaloids (−)-colchicine, imerubrine and grandirubrine. Pure Appl. Chem. 68, 539–542 (1996).
Meier, R. & Trauner, D. A synthesis of (±)-aplydactone. Angew. Chem. Int. Ed. 55, 11251–11255 (2016).
Liu, C. et al. Total synthesis of aplydactone by a conformationally controlled C−H functionalization. Angew. Chem. Int. Ed. 56, 8187–8190 (2017).
Iwasaki, K., Wan, K. K., Oppedisano, A., Crossley, S. W. M. & Shenvi, R. A. Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am. Chem. Soc. 136, 1300–1303 (2014).
Burckle, A. J., Vasilev, V. H. & Burns, N. Z. A unified approach for the enantioselective synthesis of the brominated chamigrene sesquiterpenes. Angew. Chem. Int. Ed. 55, 11476–11479 (2016).
Long, R. et al. Asymmetric total synthesis of (−)-lingzhiol via a Rh-catalysed [3+2] cycloaddition. Nat. Commun. 5, 5707 (2014).
González, D. F., Brand, J. P. & Waser, J. Ethynyl-1,2-benziodoxol-3(1 H)-one (EBX): an exceptional reagent for the ethynylation of keto, cyano, and nitro esters. Chem. Eur. J. 16, 9457–9461 (2010).
Zheng, N., Zhang, L., Gong, J. & Yang, Z. Formal total synthesis of (±)-lycojaponicumin C. Org. Lett. 19, 2921–2924 (2017).
Shao, W., Huang, J., Guo, K., Gong, J. & Yang, Z. Total synthesis of sinensilactam A. Org. Lett. 20, 1857–1860 (2018).
Gautam, K. S. & Birman, V. B. Biogenetically inspired synthesis of lingzhiol. Org. Lett. 18, 1499–1501 (2016).
Xu, Z., Wang, Q. & Zhu, J. Enantioselective total syntheses of leuconolam–leuconoxine–mersicarpine group monoterpene indole alkaloids. J. Am. Chem. Soc. 135, 19127–19130 (2013).
Xu, Z., Wang, Q. & Zhu, J. Total syntheses of (−)-mersicarpine, (−)-scholarisine G, (+)-melodinine E, (−)-leuconoxine, (−)-leuconolam, (−)-leuconodine A, (+)-leuconodine F, and (−)-leuconodine C: self-induced diastereomeric anisochronism (SIDA) phenomenon for scholarisine G and leuconodines A and C. J. Am. Chem. Soc. 137, 6712–6724 (2015).
Umehara, A., Ueda, H. & Tokuyama, H. Total syntheses of leuconoxine, leuconodine B, and melodinine E by oxidative cyclic aminal formation and diastereoselective ring-closing metathesis. Org. Lett. 16, 2526–2529 (2014).
Higuchi, K. et al. Asymmetric total synthesis of (−)-leuconoxine via chiral phosphoric acid catalyzed desymmetrization of a prochiral diester. Org. Lett. 17, 154–157 (2015).
Izgu, E. C. & Hoye, T. R. Total synthesis of (±)-leuconolam: intramolecular allylic silane addition to a maleimide carbonyl group. Chem. Sci. 4, 2262–2266 (2013).
Lu, Z., Yang, M., Chen, P., Xiong, X. & Li, A. Total synthesis of hapalindole-type natural products. Angew. Chem. Int. Ed. 53, 13840–13844 (2014).
Trost, B. M., Burns, A. C., Bartlett, M. J., Tautz, T. & Weiss, A. H. Thionium ion initiated medium-sized ring formation: the total synthesis of asteriscunolide D. J. Am. Chem. Soc. 134, 1474–1477 (2012).
Wender, P. A., Ihle, N. C. & Correia, C. R. D. Nickel-catalyzed intramolecular [4+4] cycloadditions. 4. Enantioselective total synthesis of (+)-asteriscanolide. J. Am. Chem. Soc. 110, 5904–5906 (1988).
Han, J.-C., Li, F. & Li, C.-C. Collective synthesis of humulanolides using a metathesis cascade reaction. J. Am. Chem. Soc. 136, 13610–13613 (2014).
Paquette, L. A., Tae, J., Arrington, M. P. & Sadoun, A. H. Enantioselective double Michael addition/cyclization with an oxygen-centered nucleophile as the first step in a concise synthesis of natural (+)-asteriscanolide. J. Am. Chem. Soc. 122, 2742–2748 (2000).
Lu, H.-H., Martinez, M. D. & Shenvi, R. A. An eight-step gram-scale synthesis of (−)-jiadifenolide. Nat. Chem. 7, 604–607 (2015). This paper reports an eight-step, gram-scale PGF synthesis of highly oxygenated, neurotrophic Illicium terpene (−)-jiadifenolide.
Shen, Y. et al. Protecting-group-free total synthesis of (−)-jiadifenolide: development of a [4+1] annulation toward multisubstituted tetrahydrofurans. Org. Lett. 17, 5480–5483 (2015).
Carcache, D. A. et al. Total synthesis of (±)-jiadifenin and studies directed to understanding its SAR: probing mechanistic and stereochemical issues in palladium-mediated allylation of enolate-like structures. J. Am. Chem. Soc. 128, 1016–1022 (2006).
Paterson, I., Xuan, M. & Dalby, S. M. Total synthesis of jiadifenolide. Angew. Chem. Int. Ed. 53, 7286–7289 (2014).
Xu, J., Trzoss, L., Chang, W. K. & Theodorakis, E. A. Enantioselective total synthesis of (−)-jiadifenolide. Angew. Chem. Int. Ed. 50, 3672–3676 (2011).
Xu, Z., Bao, X., Wang, Q. & Zhu, J. An enantioselective total synthesis of (−)-isoschizogamine. Angew. Chem. Int. Ed. 54, 14937–14940 (2015).
Miura, Y., Hayashi, N., Yokoshima, S. & Fukuyama, T. Total synthesis of (−)-isoschizogamine. J. Am. Chem. Soc. 134, 11995–11997 (2012).
Waldeck, A. R. & Krische, M. J. Total synthesis of cyanolide A in the absence of protecting groups, chiral auxiliaries, or premetalated carbon nucleophiles. Angew. Chem. Int. Ed. 52, 4470–4473 (2013).
Haydl, A. M. & Breit, B. Atom-economical dimerization strategy by the rhodium-catalyzed addition of carboxylic acids to allenes: protecting-group-free synthesis of clavosolide A and late-stage modification. Angew. Chem. Int. Ed. 54, 15530–15534 (2015).
Shin, I., Hong, S. & Krische, M. J. Total synthesis of swinholide A: an exposition in hydrogen-mediated C–C bond formation. J. Am. Chem. Soc. 138, 14246–14249 (2016).
Wadzinski, T. J. et al. Rapid phenolic O-glycosylation of small molecules and complex unprotected peptides in aqueous solvent. Nat. Chem. 10, 644–652 (2018).
Pelletier, G., Zwicker, A., Allen, C. L., Schepartz, A. & Miller, S. J. Aqueous glycosylation of unprotected sucrose employing glycosyl fluorides in the presence of calcium ion and trimethylamine. J. Am. Chem. Soc. 138, 3175–3182 (2016).
Downey, A. M. & Hocek, M. Strategies toward protecting group-free glycosylation through selective activation of the anomeric center. Beilstein J. Org. Chem. 13, 1239–1279 (2017).
Liu, H. & Li, X. Serine/threonine ligation: origin, mechanistic aspects, and applications. Acc. Chem. Res. 51, 1643–1655 (2018).
Mulzer, J. Trying to rationalize total synthesis. Nat. Prod. Rep. 31, 595–603 (2014).
Baran, P. S. Natural product total synthesis: as exciting as ever and here to stay. J. Am. Chem. Soc. 140, 4751–4755 (2018).
Baran, P. S. & Zbikowski, F. The charm and appeal of organic chemistry. ChemViews Magazine. https://doi.org/10.1002/chemv.201700086 (2017).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemistry: calling all engineers. Angew. Chem. Int. Ed. 57, 4149–4155 (2018).
Yang, Q.-L., Fang, P. & Mei, T.-S. Recent advances in organic electrochemical C−H functionalization. Chin. J. Chem. 36, 338–352 (2018).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Lorion, M. M., Maindan, K., Kapdi, A. R. & Ackermann, L. Heteromultimetallic catalysis for sustainable organic syntheses. Chem. Soc. Rev. 46, 7399–7420 (2017).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Zou, Y.-Q., Hörmann, F. M. & Bach, T. Iminium and enamine catalysis in enantioselective photochemical reactions. Chem. Soc. Rev. 47, 278–290 (2018).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Parasram, M. & Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: beyond conventional photosensitizers. Chem. Soc. Rev. 46, 6227–6240 (2017).
Szymkuc, S. et al. Computer-assisted synthetic planning: the end of the beginning. Angew. Chem. Int. Ed. 55, 5904–5937 (2016).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Klucznik, T. et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4, 522–532 (2018).
Acknowledgements
Financial support from the National Natural Science Foundation of China (NSFC) (21772082), Shenzhen Science and Technology Innovation (SZSTI) Commission (JCYJ20170817110515599 and KQJSCX20170728154233200), Shenzhen Peacock Plan (KQTD20150717103157174), Shenzhen Development and Reform Commission (SZDRC) Discipline Construction Program and Shenzhen Nobel Prize Scientists Laboratory Project (C17783101) is gratefully acknowledged. The authors thank J. Gong (Peking University Shenzhen Graduate School (PKUSZ)), Q. Wan (Huazhong University of Science and Technology (HUST)) and X. Li (University of Hong Kong (HKU)) for helpful discussions during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the data research, discussion, writing and editing of the Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hui, C., Chen, F., Pu, F. et al. Innovation in protecting-group-free natural product synthesis. Nat Rev Chem 3, 85–107 (2019). https://doi.org/10.1038/s41570-018-0071-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0071-1