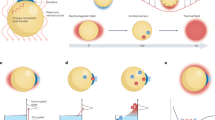
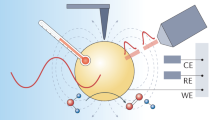
Abstract
The excitation of surface plasmons (SPs) — collective oscillation of conduction-band electrons in nanostructures — can afford photon, electron and heat energy redistribution over time and space. Making use of this ability, plasmon-enhanced molecular spectroscopy (PEMS) techniques with ultra-high sensitivity and surface selectivity have attracted much attention and have undergone considerable development over the past four decades. Recently, the development of plasmon-mediated chemical reactions (PMCRs) has shown the potential to have a large impact on the practice of chemistry. PMCRs exhibit some obvious differences from and potential advantages over traditional thermochemistry, photochemistry and photocatalysis. However, our physicochemical understanding of PMCRs is still far from complete. In this Review, we analyse the similarities and distinctive features of PEMS and PMCRs and compare PMCRs with traditional photochemical and thermochemical reactions. We then discuss how PMCRs can be improved by rationally designing and fabricating plasmonic nanostructures, selecting suitable surface and interface mediators and teaming them synergistically.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schuller, J. A. et al. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 9, 193–204 (2010).
Barnes, W. L., Dereux, A. & Ebbesen, T. W. Surface plasmon subwavelength optics. Nature 424, 824–830 (2003).
Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974).
Jeanmaire, D. L. & Van Duyne, R. P. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 84, 1–20 (1977).
Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 69, 4159–4161 (1978).
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57, 783–826 (1985).
Tian, Z. Q., Ren, B. & Wu, D. Y. Surface-enhanced Raman scattering: from noble to transition metals and from rough surfaces to ordered nanostructures. J. Phys. Chem. B 106, 9463–9483 (2002).
Ding, S. Y., You, E. M., Tian, Z. Q. & Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 46, 4042–4076 (2017).
Lal, S., Clare, S. E. & Halas, N. J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 41, 1842–1851 (2008).
Mayer, K. M. & Hafner, J. H. Localized surface plasmon resonance sensors. Chem. Rev. 111, 3828–3857 (2011).
Stiles, P. L., Dieringer, J. A., Shah, N. C. & Van Duyne, R. P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008).
Emmanuel, F. & Samuel, G. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 41, 013001 (2008).
Zhou, L. et al. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photon. 10, 393 (2016).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotech. 8, 247–251 (2013).
Linic, S., Christopher, P. & Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 10, 911–921 (2011).
Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 8, 95–103 (2014).
Baffou, G. & Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 43, 3898–3907 (2014).
Christopher, P. & Moskovits, M. Hot charge carrier transmission from plasmonic nanostructures. Annu. Rev. Phys. Chem. 68, 379–398 (2017).
Ostovar pour, S. et al. Through-space transfer of chiral information mediated by a plasmonic nanomaterial. Nat. Chem. 7, 591 (2015).
Nitzan, A. & Brus, L. E. Theoretical model for enhanced photochemistry on rough surfaces. J. Chem. Phys. 75, 2205–2214 (1981).
Chen, C. J. & Osgood, R. M. Direct observation of the local-field-enhanced surface photochemical reactions. Phys. Rev. Lett. 50, 1705–1708 (1983).
Chen, X. J., Cabello, G., Wu, D. Y. & Tian, Z. Q. Surface-enhanced Raman spectroscopy toward application in plasmonic photocatalysis on metal nanostructures. J. Photochem. Photobiol. C 21, 54–80 (2014).
Suh, J. S., Moskovits, M. & Shakhesemampour, J. Photochemical decomposition at colloid surfaces. J. Phys. Chem. 97, 1678–1683 (1993).
Suh, J. S., Jang, N. H., Jeong, D. H. & Moskovits, M. Adsorbate photochemistry on a colloid surface: phthalazine on silver. J. Phys. Chem. 100, 805–813 (1996).
Brus, L. Noble metal nanocrystals: Plasmon electron transfer photochemistry and single-molecule Raman spectroscopy. Acc. Chem. Res. 41, 1742–1749 (2008).
Huang, Y. F. et al. When the signal is not from the original molecule to be detected: chemical transformation of para-Aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 132, 9244–9246 (2010).
Wimmer, E., Fu, C. L. & Freeman, A. J. Catalytic promotion and poisoning: all-electron Local-density-functional theory of CO on Ni(001) surfaces coadsorbed with K or S. Phys. Rev. Lett. 55, 2618–2621 (1985).
Alayoglu, S., Nilekar, A. U., Mavrikakis, M. & Eichhorn, B. Ru-Pt core-shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 7, 333–338 (2008).
Ozbay, E. Plasmonics: merging photonics and electronics at nanoscale dimensions. Science 311, 189–193 (2006).
Maier, S. A. et al. Local detection of electromagnetic energy transport below the diffraction limit in metal nanoparticle plasmon waveguides. Nat. Mater. 2, 229–232 (2003).
Le Ru, E. C. & Etchegoin, P. G. in Principles of Surface-Enhanced Raman Spectroscopy Ch. 3, 121–183 (Elsevier, Amsterdam, 2009).
Maier, S. A. in Plasmonics: fundamentals and applications Ch. 5 (Springer Science & Business Media, 2007).
Link, S. & El-Sayed, M. A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 103, 8410–8426 (1999).
Hao, F. et al. Symmetry breaking in plasmonic nanocavities: subradiant LSPR sensing and a tunable Fano resonance. Nano Lett. 8, 3983–3988 (2008).
Frischkorn, C. & Wolf, M. Femtochemistry at metal surfaces: nonadiabatic reaction dynamics. Chem. Rev. 106, 4207–4233 (2006).
Khurgin, J. B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotech. 10, 2–6 (2015).
Cho, G. C., Dekorsy, T., Bakker, H. J., Hövel, R. & Kurz, H. Generation and relaxation of coherent majority plasmons. Phys. Rev. Lett. 77, 4062–4065 (1996).
Inouye, H., Tanaka, K., Tanahashi, I. & Hirao, K. Ultrafast dynamics of nonequilibrium electrons in a gold nanoparticle system. Phys. Rev. B 57, 11334–11340 (1998).
Bonn, M. et al. Phonon- versus electron-mediated desorption and oxidation of CO on Ru(0001). Science 285, 1042–1045 (1999).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotech. 10, 25–34 (2015).
Osawa, M., Ataka, K. I., Yoshii, K. & Nishikawa, Y. Surface-enhanced infrared spectroscopy: the origin of the absorption enhancement and band selection rule in the infrared spectra of molecules adsorbed on fine metal particles. Appl. Spectrosc. 47, 1497–1502 (1993).
Aroca, R. F., Ross, D. J. & Domingo, C. Surface-enhanced infrared spectroscopy. Appl. Spectrosc. 58, 324A–338A (2004).
Ortolani, M. & Limaj, O. in Handbook of Enhanced Spectroscopy 443–483 (Pan Stanford, 2015).
Tian, Z. Q., Ren, B., Li, J. F. & Yang, Z. L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. 34, 3514–3534 (2007).
Stadler, J., Schmid, T. & Zenobi, R. Developments in and practical guidelines for tip-enhanced Raman spectroscopy. Nanoscale 4, 1856–1870 (2012).
Li, J. F., Anema, J. R., Wandlowski, T. & Tian, Z. Q. Dielectric shell isolated and graphene shell isolated nanoparticle enhanced Raman spectroscopies and their applications. Chem. Soc. Rev. 44, 8399–8409 (2015).
Gruenke, N. L. et al. Ultrafast and nonlinear surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 45, 2263–2290 (2016).
Ding, S. Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Li, J. F., Li, C. Y. & Aroca, R. F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 46, 3962–3979 (2017).
Gray, S. K. Surface plasmon-enhanced spectroscopy and photochemistry. Plasmonics 2, 143–146 (2007).
Le Ru, E. C. & Etchegoin, P. G. in Principles of Surface-Enhanced Raman Spectroscopy. 185–264 (Elsevier Science, 2008).
Etchegoin, P. G. & Le Ru, E. C. in Surface Enhanced Raman Spectroscopy (ed. Schlucker, S.) 1–37 (Wiley VCH, 2010).
Ding, S. Y., Zhang, X. M., Ren, B. & Tian, Z. Q. in Encyclopedia of Analytical Chemistry (Wiley-Blackwell, 2006).
Wang, X. et al. Tip-enhanced Raman spectroscopy for surfaces and interfaces. Chem. Soc. Rev. 46, 4020–4041 (2017).
Schmid, T., Opilik, L., Blum, C. & Zenobi, R. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy: a critical review. Angew. Chem. Int. Ed. 52, 5940–5954 (2013).
Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 53, 4756–4795 (2014).
Michaels, A. M., Nirmal, M. & Brus, L. E. Surface enhanced Raman spectroscopy of individual Rhodamine 6G molecules on large Ag nanocrystals. J. Am. Chem. Soc. 121, 9932–9939 (1999).
Nie, S. & Emory, S. R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 (1997).
Zhang, R. et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 498, 82–86 (2013).
Yampolsky, S. et al. Seeing a single molecule vibrate through time-resolved coherent anti-Stokes Raman scattering. Nat. Photon. 8, 650–656 (2014).
Kerker, M., Wang, D. S. & Chew, H. Surface enhanced Raman scattering (SERS) by molecules adsorbed at spherical particles. Appl. Opt. 19, 3373–3388 (1980).
Gersten, J. & Nitzan, A. Electromagnetic theory of enhanced Raman scattering by molecules adsorbed on rough surfaces. J. Chem. Phys. 73, 3023–3037 (1980).
Aravind, P. K., Nitzan, A. & Metiu, H. The interaction between electromagnetic resonances and its role in spectroscopic studies of molecules adsorbed on colloidal particles or metal spheres. Surf. Sci. 110, 189–204 (1981).
Le Ru, E. & Etchegoin, P. in Principles of Surface-Enhanced Raman Spectroscopy Ch. 2 (Elsevier, Amsterdam, 2009).
Otto, A. Surface-enhanced Raman scattering: “Classical” and “Chemical” origins (Springer Berlin Heidelberg, 1984).
Tian, Z. Q. General Discussion. Faraday Discuss. 132, 147–158 (2006).
Wu, D. Y., Li, J. F., Ren, B. & Tian, Z. Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 37, 1025–1041 (2008).
Schatz, G. C. & Van Duyne, R. P. in Handbook of Vibrational Spectroscopy (John Wiley & Sons, Ltd, 2006).
Klessinger, M. & Michl, J. Excited States and Photochemistry of Organic Molecules (VCH Publishers, New York, 1995).
Turro, N. J. Modern Molecular Photochemistry (Univ. Science Books, Mill Valley, CA, 1991).
Karny, Z. & Zare, R. N. Infrared laser photochemistry: Evidence for heterogeneous decomposition. Chem. Phys. 23, 321–325 (1977).
Zare, R. N. Laser control of chemical reactions. Science 279, 1875–1879 (1998).
Ottosson, H. Exciting excited-state aromaticity. Nat. Chem. 4, 969 (2012).
Van Leeuwen, T., Lubbe, A. S., Štacko, P., Wezenberg, S. J. & Feringa, B. L. Dynamic control of function by light-driven molecular motors. Nat. Rev. Chem. 1, 0096 (2017).
Atwater, H. A. & Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010).
Liu, Z., Hou, W., Pavaskar, P., Aykol, M. & Cronin, S. B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 11, 1111–1116 (2011).
Ingram, D. B. & Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 133, 5202–5205 (2011).
Ueno, K. et al. Nanoparticle plasmon-assisted two-photon polymerization induced by incoherent excitation source. J. Am. Chem. Soc. 130, 6928–6929 (2008).
Murdoch, M. et al. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 3, 489 (2011).
Roger, I., Shipman, M. A. & Symes, M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017).
Honda, K. & Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Linsebigler, A. L., Lu, G. & Yates, J. T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Denzler, D. N., Frischkorn, C., Hess, C., Wolf, M. & Ertl, G. Electronic excitation and dynamic promotion of a surface reaction. Phys. Rev. Lett. 91, 226102 (2003).
Tian, Y. & Tatsuma, T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. 1810–1811 (2004).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Huang, Y. F. et al. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 53, 2353–2357 (2014).
Xie, W. & Schlücker, S. Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun. 6, 7570 (2015).
Mukherjee, S. et al. Hot-electron-induced dissociation of H2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 136, 64–67 (2014).
Martirez, J. M. P. & Carter, E. A. Excited-state N2 dissociation pathway on Fe-functionalized Au. J. Am. Chem. Soc. 139, 4390–4398 (2017).
Hou, W. et al. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 1, 929–936 (2011).
Oshikiri, T., Ueno, K. & Misawa, H. Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation. Angew. Chem. Int. Ed. 53, 9802–9805 (2014).
Zhang, N. et al. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat. Photon. 10, 473–482 (2016).
Baffou, G., Quidant, R. & Girard, C. Heat generation in plasmonic nanostructures: Influence of morphology. Appl. Phys. Lett. 94, 153109 (2009).
Baffou, G. & Quidant, R. Thermo-plasmonics: using metallic nanostructures as nano-sources of heat. Laser Photon. Rev. 7, 171–187 (2013).
Cao, L., Barsic, D. N., Guichard, A. R. & Brongersma, M. L. Plasmon-assisted local temperature control to pattern individual semiconductor nanowires and carbon nanotubes. Nano Lett. 7, 3523–3527 (2007).
Yang, Q., Xu, Q., Yu, S. H. & Jiang, H. L. Pd Nanocubes@ZIF-8: integration of plasmon-driven photothermal conversion with a metal-organic framework for efficient and selective catalysis. Angew. Chem. Int. Ed. 55, 3685–3689 (2016).
Kim, K. et al. Radiative heat transfer in the extreme near field. Nature 528, 387–391 (2015).
Hogan, N. J. et al. Nanoparticles heat through light localization. Nano Lett. 14, 4640–4645 (2014).
Govorov, A. O., Zhang, H., Demir, H. V. & Gun’ko, Y. K. Photogeneration of hot plasmonic electrons with metal nanocrystals: quantum description and potential applications. Nano Today 9, 85–101 (2014).
Sundararaman, R. et al. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 5, 5788 (2014).
Mubeen, S., Lee, J., Liu, D., Stucky, G. D. & Moskovits, M. Panchromatic photoproduction of H2 with surface plasmons. Nano Lett. 15, 2132–2136 (2015).
Harutyunyan, H. et al. Anomalous ultrafast dynamics of hot plasmonic electrons in nanostructures with hot spots. Nat. Nanotech. 10, 770–774 (2015).
Manjavacas, A., Liu, J. G., Kulkarni, V. & Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 8, 7630–7638 (2014).
Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 36, 153–166 (1997).
Furube, A., Du, L., Hara, K., Katoh, R. & Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 129, 14852–14853 (2007).
Wu, K., Chen, J., McBride, J. R. & Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 349, 632–635 (2015).
Zheng, B. Y. et al. Distinguishing between plasmon-induced and photoexcited carriers in a device geometry. Nat. Commun. 6, 7797 (2015).
Li, J. et al. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photon. 9, 601–607 (2015).
Maher, R. C. et al. Stokes/anti-Stokes anomalies under surface enhanced Raman scattering conditions. J. Chem. Phys. 120, 11746–11753 (2004).
Sushchinskii, M. M. & Rousseau, D. L. Raman spectra of molecules and crystals. Phys. Today 26, 61–62 (2008).
Sun, M., Zhang, Z., Zheng, H. & Xu, H. In-situ plasmon-driven chemical reactions revealed by high vacuum tip-enhanced Raman spectroscopy. Sci. Rep. 2, 647–650 (2012).
Zhang, Z. et al. Insights into the nature of plasmon-driven catalytic reactions revealed by HV-TERS. Nanoscale 5, 3249–3252 (2013).
Haslett, T. L., Tay, L. & Moskovits, M. Can surface-enhanced Raman scattering serve as a channel for strong optical pumping? J. Chem. Phys. 113, 1641–1646 (2000).
Brolo, A. G., Sanderson, A. C. & Smith, A. P. Ratio of the surface-enhanced anti-Stokes scattering to the surface-enhanced Stokes-Raman scattering for molecules adsorbed on a silver electrode. Phys. Rev. B 69, 045424 (2004).
Maher, R. C. et al. Resonance contributions to anti-Stokes/Stokes ratios under surface enhanced Raman scattering conditions. J. Chem. Phys. 123, 084702 (2005).
S., Y. Y. & Tamitake, I. Why and how do the shapes of surface-enhanced Raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 47, 78–88 (2016).
Itoh, T. et al. Second enhancement in surface-enhanced resonance Raman scattering revealed by an analysis of anti-Stokes and Stokes Raman spectra. Phys. Rev. B 76, 085405 (2007).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Robatjazi, H. et al. Plasmon-induced selective carbon dioxide conversion on earth-abundant aluminum-cuprous oxide antenna-reactor nanoparticles. Nat. Commun. 8, 27 (2017).
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotech. 12, 1000–1005 (2017).
Grigorenko, A. N., Polini, M. & Novoselov, K. S. Graphene plasmonics. Nat. Photon. 6, 749–758 (2012).
Lalisse, A., Tessier, G., Plain, J. & Baffou, G. Quantifying the efficiency of plasmonic materials for near-field enhancement and photothermal conversion. J. Phys. Chem. C 119, 25518–25528 (2015).
Voiry, D., Shin, H. S., Loh, K. P. & Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2, 0105 (2018).
Boltasseva, A. & Atwater, H. A. Low-loss plasmonic metamaterials. Science 331, 290–291 (2011).
Cushing, S. K. et al. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 134, 15033–15041 (2012).
Chikkaraddy, R. et al. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 535, 127–130 (2016).
Benz, F. et al. Single-molecule optomechanics in “picocavities”. Science 354, 726–729 (2016).
Schlather, A. E. et al. Hot hole photoelectrochemistry on Au@SiO2@Au nanoparticles. J. Phys. Chem. Lett. 8, 2060–2067 (2017).
Zhu, W. et al. Quantum mechanical effects in plasmonic structures with subnanometre gaps. Nat. Commun. 7, 11495 (2016).
Cortés, E. et al. Plasmonic hot electron transport drives nano-localized chemistry. Nat. Commun. 8, 14880 (2017).
Wang, P., Krasavin, A. V., Nasir, M. E., Dickson, W. & Zayats, A. V. Reactive tunnel junctions in electrically driven plasmonic nanorod metamaterials. Nat. Nanotech. 13, 159–164 (2018).
Kazuma, E., Jung, J., Ueba, H., Trenary, M. & Kim, Y. Real-space and real-time observation of a plasmon-induced chemical reaction of a single molecule. Science 360, 521–526 (2018).
Zhang, Y. et al. Visualizing coherent intermolecular dipole-dipole coupling in real space. Nature 531, 623–627 (2016).
Zhang, Y. et al. Sub-nanometre control of the coherent interaction between a single molecule and a plasmonic nanocavity. Nat. Commun. 8, 15225 (2017).
Liu, X. & Swihart, M. T. Heavily-doped colloidal semiconductor and metal oxide nanocrystals: an emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 43, 3908–3920 (2014).
V., N. G., M., S. V. & Alexandra, B. Alternative plasmonic materials: beyond gold and silver. Adv. Mater. 25, 3264–3294 (2013).
Stensitzki, T. et al. Acceleration of a ground-state reaction by selective femtosecond-infrared-laser-pulse excitation. Nat. Chem. 10, 126 (2018).
Petek, H. Photoexcitation of adsorbates on metal surfaces: one-step or three-step. J. Chem. Phys. 137, 091704 (2012).
Acknowledgements
The authors are deeply grateful to M. Moskovits for his very helpful suggestions and careful academic and English editing of the manuscript. This work is financially supported by the National Natural Science Foundation of China (21533006, 21621091, 91427304 and 21403180) and the Ministry of Science and Technology of China (2015CB932300).
Reviewers information
Nature Reviews Chemistry thanks R. Aroca, S. Schlücker and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Z-Q.T. conceived the outline. C.Z., X-J.C. and Z-Q.T. wrote the manuscript. J.Y. supplied the calculation in Figure 1. All authors contributed to discussions, editing and corrections. C.Z. and Z-Q.T. revised the manuscript before the final submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Optical diffraction limit
-
The fundamental maximum of the spatial resolution of an optical system that is due to diffraction.
- Landau damping
-
The damping effect of longitudinal space charge waves in plasma or a similar environment. Landau damping occurs because of the energy exchange between an electromagnetic wave and particles (for example, electrons) in the plasma, which can interact strongly with the wave. In a surface plasmon system, the Landau damping process represents the direct absorption of a photon assisted by the surface plasmon momentum, creating a hot hole and a hot electron.
- Fermi–Dirac distribution
-
The distribution of particles over energy states in systems consisting of many identical particles that obey the Pauli exclusion principle.
- Electrostatic approximation
-
The assumption that the phase of the harmonically oscillating electromagnetic field is practically constant over the particle volume, so that one can calculate the spatial field distribution by assuming the simplified problem of a particle in an electrostatic field.
- Drude model
-
A model used to explain the transport properties of electrons in materials in which the microscopic behaviour of electrons in a solid is treated classically. It is the basic model used in the study of optical properties of different materials and is commonly used to explain the dielectric function of plasmonic nanostructures.
- Fermi level
-
The highest energy level that an electron can fill in the solid state at absolute zero temperature.
- Förster resonance energy transfer
-
(FRET). A mechanism describing the energy transfer between two light-sensitive dipoles, in which energy non-radiatively transfers from a blueshifted emitter to a redshifted absorber through dipole–dipole coupling.
Rights and permissions
About this article
Cite this article
Zhan, C., Chen, XJ., Yi, J. et al. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat Rev Chem 2, 216–230 (2018). https://doi.org/10.1038/s41570-018-0031-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0031-9
This article is cited by
-
Manipulating submolecular-scale phototautomerization
Science China Materials (2024)
-
Near-infrared-featured broadband CO2 reduction with water to hydrocarbons by surface plasmon
Nature Communications (2023)
-
Electrochemical surface-enhanced Raman spectroscopy
Nature Reviews Methods Primers (2023)
-
Plasmon-mediated chemical reactions
Nature Reviews Methods Primers (2023)
-
Strong synergy between gold nanoparticles and cobalt porphyrin induces highly efficient photocatalytic hydrogen evolution
Nature Communications (2023)