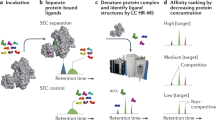
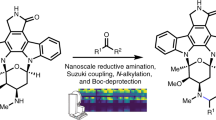
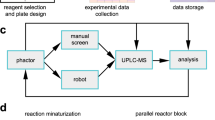
Abstract
The discovery of bioactive small molecules is generally driven via iterative design–make–purify–test cycles. Automation is routinely harnessed at individual stages of these cycles to increase the productivity of drug discovery. Here, we describe recent progress to automate and integrate two or more adjacent stages within discovery workflows. Examples of such technologies include microfluidics, liquid-handling robotics and affinity-selection mass spectrometry. The value of integrated technologies is illustrated in the context of specific case studies in which modulators of targets, such as protein kinases, nuclear hormone receptors and protein–protein interactions, were discovered. We note that to maximize impact on the productivity of discovery, each of the integrated stages would need to have both high and matched throughput. We also consider the longer-term goal of realizing the fully autonomous discovery of bioactive small molecules through the integration and automation of all stages of discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010).
Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 17, 97–113 (2017).
Macarron, R. et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 10, 188–195 (2011).
Ley, S. V., Fitzpatrick, D. E., Ingham, R. J. & Myers, R. M. Organic synthesis: march of the machines. Angew. Chem. Int. Ed. 54, 3449–3464 (2015).
Scott, D. E., Bayly, A. R., Abell, C. & Skidmore, J. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug Discov. 15, 533–550 (2016).
Polishchuk, P. G., Madzhidov, T. I. & Varnek, A. Estimation of the size of drug-like chemical space based on GDB-17 data. J. Comput. Aided Mol. Des. 27, 675–679 (2013).
Lipkus, A. H. et al. Structural diversity of organic chemistry. A scaffold analysis of the CAS Registry. J. Org. Chem. 73, 4443–4451 (2008).
Langdon, S. R., Brown, N. & Blagg, J. Scaffold diversity of exemplified medicinal chemistry space. J. Chem. Inf. Model. 51, 2174–2185 (2011).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Lipinski, C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug. Discov. Today Technol. 1, 337–341 (2004).
Foley, D. J., Nelson, A. & Marsden, S. P. Evaluating new chemistry to drive molecular discovery: fit for purpose? Angew. Chem. Int. Ed. 55, 13650–13657 (2016).
Walters, W. P., Green, J., Weiss, J. R. & Murcko, M. A. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 54, 6405–6416 (2011).
Schneider, N., Lowe, D. M., Sayle, R. A., Tarselli, M. A. & Landrum, G. A. Big data from pharmaceutical patents: a computational analysis of medicinal chemists’ bread and butter. J. Med. Chem. 59, 4385–4402 (2016).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Brown, D. G. & Bostrom, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Cooper, T. W., Campbell, I. B. & Macdonald, S. J. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew, Chem. Int. Ed. 49, 8082–8091 (2010).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Goodnow, R. A. Jr, Dumelin, C. E. & Keefe, A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131–147 (2016).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Ley, S. V. et al. Multi-step organic synthesis using solid-supported reagents and scavengers: a new paradigm in chemical library generation. J. Chem. Soc. Perkin Trans. 1, 3815–4195 (2000).
Carpintero, M., Cifuentes, M., Ferritto, R., Haro, R. & Toledo, M. A. Automated liquid-liquid extraction workstation for library synthesis and its use in the parallel and chromatography-free synthesis of 2-alkyl-3-alkyl-4-(3H)-quinazolinones. J. Comb. Chem. 9, 818–822 (2007).
Ghislieri, D., Gilmore, K. & Seeberger, P. H. Chemical assembly systems: layered control for divergent, continuous, multistep syntheses of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 54, 678–682 (2015).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 51, 1114–1122 (2012).
Hwang, Y. J. J. et al. A segmented flow platform for on-demand medicinal chemistry and compound synthesis in oscillating droplets. Chem. Comm. 53, 6649–6652 (2017).
Reizman, B. J., Wang, Y. M., Buchwald, S. L. & Jensen, K. F. Suzuki-Miyaura cross-coupling optimization enabled by automated feedback. React. Chem. Eng. 1, 658–666 (2016).
Cernak, T. et al. Microscale high-throughput experimentation as an enabling technology in drug discovery: application in the discovery of (Piperidinyl)pyridinyl-1H-benzimidazole diacylglycerol acyltransferase 1 inhibitors. J. Med. Chem. 60, 3594–3605 (2017).
Buitrago Santanilla, A. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Perera, D. et al. A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow. Science 359, 429–434 (2018).
Murray, P. M., Tyler, S. N. G. & Moseley, J. D. Beyond the numbers: charting chemical reaction space. Org. Process Res. Dev. 17, 40–46 (2013).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Qin, T. et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Troshin, K. & Hartwig, J. F. Snap deconvolution: an informatics approach to high-throughput discovery of catalytic reactions. Science 357, 175–181 (2017).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Hawkes, S. Y. F., Chapela, M. J. V. & Montembault, M. Leveraging the advantages offered by microfluidics to enhance the drug discovery process. QSAR Comb. Sci. 24, 712–721 (2005).
Wang, J. et al. Integrated microfluidics for parallel screening of an in situ click chemistry library. Angew. Chem. Int. Ed. 45, 5276–5281 (2006).
Baranczak, A. et al. Integrated platform for expedited synthesis−purification−testing of small molecule libraries. ACS Med. Chem. Lett. 8, 461–465 (2017).
Czechtizky, W. et al. Integrated synthesis and testing of substituted xanthine based DPP4 inhibitors: application to drug discovery. ACS Med. Chem. Lett. 4, 768–772 (2013).
Guetzoyan, L. et al. Machine-assisted synthesis of modulators of the histone reader BRD9 using flow methods of chemistry and frontal affinity chromatography. Med. Chem. Commun. 5, 540 (2014).
Guetzoyan, L., Nikbin, N., Bexandale, I. R. & Ley, S. V. Flow chemistry synthesis of zolpidem, alpidem and other GABAA agonists and their biological evaluation through the use of in-line frontal affinity chromatography. Chem. Sci. 4, 764–769 (2013).
Werner, M. et al. Seamless integration of dose-response screening and flow chemistry: efficient generation of structure–activity relationship data of b-secretase (BACE1) inhibitors. Angew. Chem. Int. Ed. 53, 1704–1708 (2014).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Desai, B. et al. Rapid discovery of a novel series of Abl kinase inhibitors by application of an integrated microfluidic synthesis and screening platform. J. Med. Chem. 56, 3033–3047 (2013).
Reker, D. & Schneider, G. Active-learning strategies in computer-assisted drug discovery. Drug Discov. Today 20, 458–465 (2015).
Murray, J. B., Roughley, S. D., Matassova, N. & Brough, P. A. Off-rate screening (ORS) by surface plasmon resonance. An efficient method to kinetically sample hit to lead chemical space from unpurified reaction products. J. Med. Chem. 57, 2845–2850 (2014).
Maplestone, R. A., Stone, M. J. & Williams, D. H. The evolutionary role of secondary metabolites — a review. Gene 115, 151–157 (1992).
Firn, R. D. & Jones, C. G. Natural products — a simple model to explain chemical diversity. Nat. Prod. Rep. 20, 382 (2003).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod 75, 311–335 (2012).
Karageorgis, G., Warriner, S. & Nelson, A. Efficient discovery of bioactive scaffolds by activity-directed synthesis. Nat. Chem. 6, 872–876 (2014).
Karageorgis, G., Dow, M., Aimon, A., Warriner, S. & Nelson, A. Activity-directed synthesis with intermolecular reactions: development of a fragment into a range of androgen receptor agonists. Angew. Chem. Int. Ed. 54, 13538–13544 (2015).
Huang, Y. L. & Bode, J. W. Synthetic fermentation of bioactive non-ribosomal peptides without organisms, enzymes or reagents. Nat. Chem. 6, 877–884 (2014).
Mondal, M. & Hirsch, A. K. H. Dynamic combinatorial chemistry: a tool to facilitate the identification of inhibitors for protein targets. Chem. Soc. Rev. 44, 2455–2488 (2015).
Coley, C. W., Barzilay, R., Jaakkola, T. S., Green, W. H. & Jensen, K. F. Prediction of organic reaction outcomes using machine learning. ACS Cent. Sci. 3, 434–443 (2017).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Yoshida, M. et al. Using evolutionary algorithms and machine learning to explore sequence space for the discovery of antimicrobial peptides. Chem 4, 533–543 (2018).
Pickett, S. D., Green, D. V. S., Hunt, D. L., Pardoe, D. A. & Hughes, I. Automated lead optimization of MMP-12 inhibitors using a genetic algorithm. ACS Med. Chem. Lett. 2, 28–33 (2011).
Acknowledgements
The authors thank the Engineering and Physical Sciences Research Council (EPSRC; EP/N025652/1) for support.
Reviewer information
Nature Reviews Chemistry thanks D. Brown, D. Winkler and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
S.C. and S.L. researched data for the article. All authors made substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chow, S., Liver, S. & Nelson, A. Streamlining bioactive molecular discovery through integration and automation. Nat Rev Chem 2, 174–183 (2018). https://doi.org/10.1038/s41570-018-0025-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0025-7
This article is cited by
-
Future medicinal chemists experience flow chemistry: optimization by experimental design of the limiting synthetic step to the antifungal drug econazole nitrate
Journal of Flow Chemistry (2021)
-
A droplet microfluidic platform for high-throughput photochemical reaction discovery
Nature Communications (2020)