Abstract
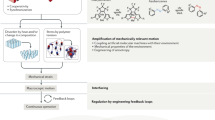
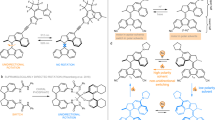
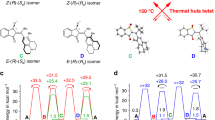
The field of dynamic functional molecular systems has progressed enormously over the past few decades. By coupling the mechanical properties of molecular switches and motors to chemical and biological processes, exceptional control of function has been attained. Overcrowded alkene-based light-driven molecular motors are very attractive in this respect owing to their unique multistate photochemically and thermally induced switching processes and their helical chirality inversion in each switching step. However, extending our control over properties from the molecular scale to larger length scales is still a fundamental challenge. In this Perspective, we discuss recent developments that address this challenge, ranging from the application of these motors in catalysis and synthetic materials to the control of biological properties. We may now be positioned at the dawn of a new era in which artificial molecular motors are able to perform programmed tasks and dynamic functions akin to the biological machines that are found in daily life.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Russew, M. M. & Hecht, S. Photoswitches: from molecules to materials. Adv. Mater. 22, 3348–3360 (2010).
Klajn, R., Stoddart, J. F. & Grzybowski, B. A. Nanoparticles functionalized with reversible molecular and supramolecular switches. Chem. Soc. Rev. 39, 2203–2237 (2010).
Sauvage, J.-P. & Gaspard, P. From Non-Covalent Assemblies to Molecular Machines (Wiley, 2011).
Qu, D.-H., Wang, Q.-C., Zhang, Q.-W., Ma, X. & Tian, H. Photoresponsive host-guest functional systems. Chem. Rev. 115, 7543–7588 (2015).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Feringa, B. L. The art of building small: from molecular switches to molecular motors. J. Org. Chem. 72, 6635–6652 (2007).
Neilson, B. M. & Bielawski, C. W. Illuminating photoswitchable catalysis. ACS Catal. 3, 1874–1885 (2013).
Ueno, A., Takahashi, K. & Osa, T. Photoregulation of catalytic activity of β-cyclodextrin by an azo inhibitor. J. Chem. Soc. Chem. Commun.http:dx.doi.org/10.1039/C39800000837 (1980).
Stoll, R. S. & Hecht, S. Artificial light-gated catalyst systems. Angew. Chem. Int. Ed. 49, 5054–5075 (2010).
Blanco, V., Leigh, D. A. & Marcos, V. Artificial switchable catalysts. Chem. Soc. Rev. 44, 5341–5370 (2015).
Vlatkovic, M., Collins, B. S. L. & Feringa, B. L. Dynamic responsive systems for catalytic function. Chemistry 22, 17080–17111 (2016).
Wang, J. & Feringa, B. L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 331, 1429–1432 (2011).
Vlatkovic´, M., Bernardi, L., Otten, E. & Feringa, B. L. Dual stereocontrol over the Henry reaction using a light- and heat-triggered organocatalyst. Chem. Commun. 50, 7773–7775 (2014).
Zhao, D., Neubauer, T. M. & Feringa, B. L. Dynamic control of chirality in phosphine ligands for enantioselective catalysis. Nat. Commun. 6, 6652 (2015).
Chen, C.-T., Tsai, C.-C., Tsou, P., Huang, G.-T. & Yu, C.-H. Enantiodivergent Steglich rearrangement of O-carboxylazlactones catalyzed by a chirality switchable helicene containing a 4-aminopyridine unit. Chem. Sci. 8, 524–529 (2017).
Teator, A. J., Lastovickova, D. M. & Bielawski, C. W. Switchable polymerization catalysts. Chem. Rev. 116, 1969–1992 (2016).
Yashima, E. et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 116, 13752–13990 (2016).
Green, M. M. et al. A helical polymer with cooperative response to chiral information. Science 268, 1860–1866 (1995).
Palmans, A. R. A. & Meijer, E. W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 46, 8948–8968 (2007).
Pijper, D. & Feringa, B. L. Control of dynamic helicity at the macro- and supramolecular level. Soft Matter 4, 1349–1372 (2008).
Pijper, D. & Feringa, B. L. Molecular transmission: controlling the twist sense of a helical polymer with a single light-driven molecular motor. Angew. Chem. Int. Ed. 46, 3693–3696 (2007).
Zhao, D., van Leeuwen, T., Cheng, J. & Feringa, B. L. Dynamic control of chirality and self-assembly of double-stranded helicates with light. Nat. Chem. 9, 200–256 (2017).
Lehn, J. M. et al. Spontaneous assembly of double-stranded helicates from oligobipyridine ligands and copper(i) cations: structure of an inorganic double helix. Proc. Natl Acad. Sci. USA 84, 2565–2569 (1987).
Eelkema, R. & Feringa, B. L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 4, 3729–3745 (2006).
Pieraccini, S., Masiero, S., Ferrarini, A. & Spada, G. P. Chirality transfer across length-scales in nematic liquid crystals: fundamentals and applications. Chem. Soc. Rev. 40, 258–271 (2011).
Katsonis, N., Lacaze, E. & Ferrarini, A. Controlling chirality with helix inversion in cholesteric liquid crystals. J. Mater. Chem. 22, 7088–7097 (2012).
Wang, J. & Li, Q. Light-driven chiral molecular switches or motors in liquid crystals. Adv. Mater. 24, 1926–1945 (2012).
Eelkema, R. et al. Molecular machines: nanomotor rotates microscale objects. Nature 440, 163 (2006).
Eelkema, R. et al. Rotational reorganization of doped cholesteric liquid crystalline films. J. Am. Chem. Soc. 128, 14397–14407 (2006).
Wezenberg, S. J., Croisetu, C. M., Stuart, M. C. A. & Feringa, B. L. Reversible gel-sol photoswitching with an overcrowded alkene-based bis-urea supergelator. Chem. Sci. 7, 4341–4346 (2016).
Chen, C. T., Chen, C. H. & Ong, T. G. Complementary helicity interchange of optically switchable supramolecular-enantiomeric helicenes with (−)-gel-sol-(+)-gel transition ternary logic. J. Am. Chem. Soc. 135, 5294–5297 (2013).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Webber, M. J., Appel, E. A. & Meijer, E. W. & Langer, R. Supramolecular biomaterials. Nat. Mater. 15, 13–26 (2016).
van Dijken, D. J., Chen, J., Stuart, M. C. A., Hou, L. & Feringa, B. L. Amphiphilic molecular motors for responsive aggregation in water. J. Am. Chem. Soc. 138, 660–669 (2016).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat. Nanotechnol. 10, 161–165 (2015).
Foy, J. T. et al. Dual-light control of nanomachines that integrate motor and modulator subunits. Nat. Nanotechnol. 12, 540–545 (2017).
Katsonis, N., Lubomska, M., Pollard, M. M., Feringa, B. L. & Rudolf, P. Synthetic light-activated molecular switches and motors on surfaces. Prog. Surf. Sci. 82, 407–434 (2007).
van Delden, R. A. et al. Unidirectional molecular motor on a gold surface. Nature 437, 1337–1430 (2005).
Chen, K.-Y. et al. Control of surface wettability using tripodal light-activated molecular motors. J. Am. Chem. Soc. 136, 3219–3224 (2014).
Berná, J. et al. Macroscopic transport by synthetic molecular machines. Nat. Mater. 4, 704–710 (2005).
Szymański, W., Beierle, J. M., Kistemaker, H. A. V., Velema, W. A. & Feringa, B. L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 113, 6114–6178 (2013).
Broichhagen, J., Frank, J. A. & Trauner, D. A. Roadmap to success in photopharmacology. Acc. Chem. Res. 48, 1947–1960 (2015).
Velema, W. A., Szymanski, W. & Feringa, B. L. Photopharmacology: beyond proof of principle. J. Am. Chem. Soc. 136, 2178–2191 (2014).
Lerch, M. M., Hansen, M. J., van Dam, G. M., Szymanski, W. & Feringa, B. L. Emerging targets in photopharmacology. Angew. Chem. Int. Ed. 55, 10978–10999 (2016).
van Delden, R. A., Koumura, N., Schoevaars, A., Meetsma, A. & Feringa, B. L. A donor–acceptor substituted molecular motor: unidirectional rotation driven by visible light. Org. Biomol. Chem. 1, 33–35 (2003).
Cnossen, A. et al. Driving unidirectional molecular rotary motors with visible light by intra- and intermolecular energy transfer from palladium porphyrin. J. Am. Chem. Soc. 134, 17613–17619 (2012).
Wezenberg, S. J., Chen, K.-Y. & Feringa, B. L. Visible-light-driven photoisomerization and increased rotation speed of a molecular motor acting as a ligand in a ruthenium(ii) complex. Angew. Chem. Int. Ed. 54, 11457–11461 (2015).
Yoon, I., Li, J. Z. & Shim, Y. K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 46, 7–23 (2013).
Poloni, C., Stuart, M. C. A., van der Meulen, P., Szymanski, W. & Feringa, B. L. Light and heat control over secondary structure and amyloid-like fiber formation in an overcrowded-alkene-modified Trp zipper. Chem. Sci. 6, 7311–7318 (2015).
Wezenberg, S. J., Vlatkovic, M., Kistemaker, J. C. M. & Feringa, B. L. Multi-state regulation of the dihydrogen phosphate binding affinity to a light- and heat-responsive bis-urea receptor. J. Am. Chem. Soc. 136, 16784–16787 (2014).
Vlatkovic, M., Feringa, B. L. & Wezenberg, S. J. Dynamic inversion of stereoselective phosphate binding to a bisurea receptor controlled by light and heat. Angew. Chem. Int. Ed. 55, 1001–1004 (2016).
Filatov, M. & Olivucci, M. Designing conical intersections for light-driven single molecule rotary motors: from precessional to axial motion. J. Org. Chem. 79, 3587–3600 (2014).
Faulkner, A., van Leeuwen, T., Feringa, B. L. & Wezenberg, S. J. Allosteric regulation of the rotational speed in a light-driven molecular motor. J. Am. Chem. Soc. 138, 13597–13603 (2016).
Pramanik, S. & Aprahamian, I. Hydrazone switch-based negative feedback loop. J. Am. Chem. Soc. 138, 15142–15145 (2016).
Qu, D.-H. & Feringa, B. L. Controlling molecular rotary motion with a self-complexing lock. Angew. Chem. Int. Ed. 49, 1107–1110 (2010).
Zhu, K., O’Keefe, C. A., Vukotic, V. N., Schurko, R. W. & Loeb, S. J. A molecular shuttle that operates inside a metal–organic framework. Nat. Chem. 7, 514–519 (2015).
Kaleta, J. et al. Surface inclusion of unidirectional molecular motors in hexagonal tris(o-phenylene)cyclotriphosphazene. J. Am. Chem. Soc. 139, 10486–10498 (2017).
Štacko, P. et al. Locked synchronous rotor motion in a molecular motor. Science 356, 964–968 (2017).
Green, J. E. et al. 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 445, 414–417 (2007).
Acknowledgements
The authors gratefully acknowledge generous support from NanoNed, The Netherlands Organization for Scientific Research (NWO-CW Top grant to B.L.F. and NWO-CW Veni grant No. 722.014.006 to S.J.W.), the Royal Netherlands Academy of Arts and Sciences (KNAW), the Ministry of Education, Culture and Science (Gravitation programme 024.001.035) and the European Research Council (Advanced Investigator Grant No. 694345 to B.L.F.).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the article, discussing the content and writing and editing of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
van Leeuwen, T., Lubbe, A., Štacko, P. et al. Dynamic control of function by light-driven molecular motors. Nat Rev Chem 1, 0096 (2017). https://doi.org/10.1038/s41570-017-0096
Published:
DOI: https://doi.org/10.1038/s41570-017-0096
This article is cited by
-
Photo-responsive functional materials based on light-driven molecular motors
Light: Science & Applications (2024)
-
A photochemical method to evidence directional molecular motions
Nature Communications (2023)
-
Cation controlled rotation in anionic pillar[5]arenes and its application for fluorescence switch
Nature Communications (2023)
-
Switchable aqueous catalytic systems for organic transformations
Communications Chemistry (2022)
-
Photogearing as a concept for translation of precise motions at the nanoscale
Nature Chemistry (2022)