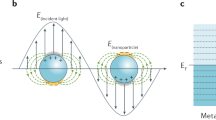
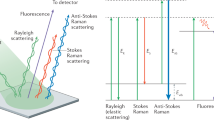
Abstract
Surface-enhanced Raman scattering (SERS) is of interest for biomedical analysis and imaging because of its sensitivity, specificity and multiplexing capabilities. The successful application of SERS for in vivo biosensing requires probes to be biocompatible and procedures to be minimally invasive, challenges that have respectively been met by developing new nanoprobes and instrumentation. This Review presents recent developments in these areas, describing case studies in which sensors have been implemented, as well as outlining shortcomings that must be addressed before SERS sees clinical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Raman, C. V. & Krishnan, K. S. A new type of secondary radiation. Nature 121, 501–502 (1928).
Raman, C. V. A new radiation. Indian J. Phys. 2, 387–398 (1928).
Diem, M., Romeo, M., Boydston-White, S., Miljkovic´, M. & Matthäus, C. A decade of vibrational micro-spectroscopy of human cells and tissue (1994–2004). Analyst 129, 880–885 (2004).
Kong, K., Kendall, C., Stone, N. & Notingher, I. Raman spectroscopy for medical diagnostics—from in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 89, 121–134 (2015).
Byrne, H. J. et al. Spectropathology for the next generation: quo vadis? Analyst 140, 2066–2073 (2015).
Stone, N., Kendall, C., Smith, J., Crow, P. & Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 126, 141–157 (2004).
Jamieson, L. E. & Byrne, H. J. Vibrational spectroscopy as a tool for studying drug–cell interaction: could high throughput vibrational spectroscopic screening improve drug development? Vib. Spectrosc. 91, 16–30 (2017).
Farhane, Z., Bonnier, F., Casey, A. & Byrne, H. J. Raman micro spectroscopy for in vitro drug screening: subcellular localisation and interactions of doxorubicin. Analyst 140, 4212–4223 (2015).
Matthäus, C., Kale, A., Chernenko, T., Torchilin, V. & Diem, M. New ways of imaging uptake and intracellular fate of liposomal drug carrier systems inside individual cells, based on Raman microscopy. Mol. Pharm. 5, 287–293 (2008).
Atkins, P., de Paula, J. & Friedman, R. Physical Chemistry: Quanta, Matter, and Change (Oxford Univ. Press, 2013).
Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974).
Bergman, I. et al. General discussion. Faraday Discuss. Chem. Soc. 56, 152–170 (1973).
Jeanmaire, D. L. & Van Duyne, R. P. Surface Raman spectroelectrochemistry: part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 84, 1–20 (1977).
Yang, S., Dai, X., Stogin, B. B. & Wong, T.-S. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl Acad. Sci. USA 113, 268–273 (2016).
Kneipp, J., Kneipp, H. & Kneipp, K. SERS—a single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 37, 1052–1060 (2008).
Smith, E. & Dent, G. Modern Raman Spectroscopy: A Practical Approach 113–133 (Wiley, 2013).
Campion, A. & Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 27, 241–250 (1998).
Schlücker, S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 53, 4756–4795 (2014).
Stiles, P. L., Dieringer, J. A., Shah, N. C. & Van Duyne, R. R. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008).
Vo-Dinh, T., Hiromoto, M. Y. K., Begun, G. M. & Moody, R. L. Surface-enhanced Raman spectrometry for trace organic analysis. Anal. Chem. 56, 1667–1670 (1984).
Rohr, T. E., Cotton, T., Fan, N. & Tarcha, P. J. Immunoassay employing surface-enhanced Raman spectroscopy. Anal. Biochem. 182, 388–398 (1989).
Vo-Dinh, T., Houck, K. & Stokes, D. L. Surface-enhanced Raman gene probes. Anal. Chem. 66, 3379–3383 (1994).
Graham, D., Mallinder, B. J. & Smith, W. E. Surface-enhanced resonance Raman scattering as a novel method of DNA discrimination. Angew. Chem. Int. Ed. 39, 1061–1063 (2000).
Faulds, K., Jarvis, R., Smith, W. E., Graham, D. & Goodacre, R. Multiplexed detection of six labelled oligonucleotides using surface enhanced resonance Raman scattering (SERRS). Analyst 133, 1505–1512 (2008).
Gracie, K. et al. Simultaneous detection and quantification of three bacterial meningitis pathogens by SERS. Chem. Sci. 5, 1030–1040 (2014).
Song, J., Zhou, J. & Duan, H. Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J. Am. Chem. Soc. 134, 13458–13469 (2012).
Nabiev, I. R., Morjani, H. & Manfait, M. Selective analysis of antitumor drug interaction with living cancer cells as probed by surface-enhanced Raman spectroscopy. Eur. Biophys. J. 19, 311–316 (1991).
Schlücker, S. et al. Immuno-Raman microspectroscopy: in situ detection of antigens in tissue specimens by surface-enhanced Raman scattering. J. Raman Spectrosc. 37, 719–721 (2006).
Stuart, D. A. et al. In vivo glucose measurement by surface-enhanced Raman spectroscopy. Anal. Chem. 78, 7211–7215 (2006). This first report of in vivo SERS, here applied to monitoring glucose concentrations in a live mouse using a SAM–AgFON SERS sensor and an optical window.
White, P. L. et al. Evaluation of a commercially developed semiautomated PCR–SERS assay for the diagnosis of invasive fungal disease. J. Clin. Microbiol. 52, 3536–3543 (2014).
Leask, F. Renishaw Diagnostics announces CE marking of Rendx Multiplex Assay System and Fungiplex Assay. SelectSciencehttp://www.selectscience.net/industry-news/renishaw-diagnostics-announces-ce-marking-of-rendx-multiplex-assay-system-and-fungiplex-assay/?artID=38483 (2015).
Graham, D., Faulds, K., Smith, W. E. & Ricketts, A. Identification of nucleic acid sequences. WO 2009022125A1 (2009).
Cai, W., Gao, T., Hong, H. & Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008:1, 17–32 (2008).
Caspers, P. J., Lucassen, G. W. & Puppels, G. J. Combined in vivo confocal Raman spectroscopy and confocal microscopy of human skin. Biophys. J. 85, 572–580 (2003).
Boncheva, M., de Sterke, J., Caspers, P. J. & Puppels, G. J. Depth profiling of Stratum corneum hydration in vivo: a comparison between conductance and confocal Raman spectroscopic measurements. Exp. Dermatol. 18, 870–876 (2009).
Mahadevan-Jansen, A., Mitchell, M. F., Ramanujam, N., Utzinger, U. & Richards-Kortum, R. Development of a fiber optic probe to measure NIR Raman spectra of cervical tissue in vivo. Photochem. Photobiol. 68, 427–431 (1998).
Draga, R. O. et al. In vivo bladder cancer diagnosis by high-volume Raman spectroscopy. Anal. Chem. 82, 5993–5999 (2010).
Shim, M. G., Song, L. M., Marcon, N. E. & Wilson, B. C. In vivo near-infrared Raman spectroscopy: demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem. Photobiol. 72, 146–150 (2000).
Haka, A. S. et al. In vivo margin assessment during partial mastectomy breast surgery using Raman spectroscopy. Cancer Res. 66, 3317–3322 (2006).
Jermyn, M. et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 7, 274ra219 (2015).
Walsh, F. Laser detects brain tumour cells during surgery. BBChttp://www.bbc.co.uk/news/health-34041863 (2015).
UK hospital to trial Raman probe for brain tumors. optics.orghttp://optics.org/news/6/1/18 (2015).
Evans, C. L. et al. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl Acad. Sci. USA 102, 16807–16812 (2005).
Fu, Y., Huff, T. B., Wang, H. W., Wang, H. & Cheng, J. X. Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt. Express 16, 19396–19409 (2008).
Ji, M. et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 5, 201ra119 (2013).
Saar, B. G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Yuen, J. M., Shah, N. C., Walsh, J. T., Glucksberg, M. R. & Van Duyne, R. P. Transcutaneous glucose sensing by surface-enhanced spatially offset Raman spectroscopy in a rat model. Anal. Chem. 82, 8382–8385 (2010).
Oseledchyk, A., Andreou, C., Wall, M. A. & Kircher, M. F. Folate-targeted surface-enhanced resonance Raman scattering nanoprobe ratiometry for detection of microscopic ovarian cancer. ACS Nano 11, 1488–1497 (2017).
Sharma, B. et al. Bisboronic acids for selective, physiologically relevant direct glucose sensing with surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 138, 13952–13959 (2016).
Ma, K. et al. In vivo, transcutaneous glucose sensing using surface-enhanced spatially offset Raman spectroscopy: multiple rats, improved hypoglycemic accuracy, low incident power, and continuous monitoring for greater than 17 days. Anal. Chem. 83, 9146–9152 (2011).
Dinish, U. S., Balasundaram, G., Chang, Y.-T. & Olivo, M. Actively targeted in vivo multiplex detection of intrinsic cancer biomarkers using biocompatible SERS nanotags. Sci. Rep. 4, 4075 (2014).
Samanta, A. et al. Ultrasensitive near-infrared Raman reporters for SERS-based in vivo cancer detection. Angew. Chem. Int. Ed. 50, 6089–6092 (2011). In this study, a library of tricarbocyanine reporters, optimized for NIR excitation, was synthesized and screened. Of these, the 1,2-dithiolane CyNAMLA-381 was used for sensitive and specific detection of tumours.
Wang, Y. et al. Quantitative molecular phenotyping with topically applied SERS nanoparticles for intraoperative guidance of breast cancer lumpectomy. Sci. Rep. 6, 21242 (2016).
Tian, F. et al. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials 106, 87–97 (2016).
Papadopoulou, E. & Bell, S. E. J. Label-free detection of single-base mismatches in DNA by surface-enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 50, 9058–9061 (2011).
Pavel, I. et al. Label-free SERS detection of small proteins modified to act as bifunctional linkers. J. Phys. Chem. C 112, 4880–4883 (2008).
Graham, D. et al. Synthesis of novel monoazo benzotriazole dyes specifically for surface enhanced resonance Raman scattering. Chem. Commun. 1187–1188 (1998).
Schütz, M., Müller, C. I., Salehi, M., Lambert, C. & Schlücker, S. Design and synthesis of Raman reporter molecules for tissue imaging by immuno-SERS microscopy. J. Biophoton. 4, 453–463 (2011).
Faulds, K., Smith, W. E. & Graham, D. DNA detection by surface enhanced resonance Raman scattering (SERRS). Analyst 130, 1125–1131 (2005).
Drescher, D. & Kneipp, J. Nanomaterials in complex biological systems: insights from Raman spectroscopy. Chem. Soc. Rev. 41, 5780–5799 (2012).
Taylor, J., Huefner, A., Li, L., Wingfield, J. & Mahajan, S. Nanoparticles and intracellular applications of surface-enhanced Raman spectroscopy. Analyst 141, 5037–5055 (2016).
Alkilany, A. M. & Murphy, C. J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart. Res. 12, 2313–2333 (2010).
Sakhtianchi, R. et al. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv. Colloid Interface Sci. 201, 18–29 (2013).
Dykman, L. A. & Khlebtsov, N. G. Uptake of engineered gold nanoparticles into mammalian cells. Chem. Rev. 114, 1258–1288 (2014).
Zhang, X.-D. et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomed. 6, 2071–2081 (2011).
Bhamidipati, M. & Fabris, L. Multiparametric assessment of gold nanoparticle cytotoxicity in cancerous and healthy cells: the role of size, shape, and surface chemistry. Bioconjug. Chem. 28, 449–460 (2017).
Schlinkert, P. et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J. Nanobiotechnol. 13, 1 (2015).
Falagan-Lotsch, P., Grzincic, E. M. & Murphy, C. J. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc. Natl Acad. Sci. USA 113, 13318–13323 (2016).
Albanese, A. & Chan, W. C. W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 5, 5478–5489 (2011).
Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 19, 316–317 (2001).
Stuart, D. A. et al. Glucose sensing using near-infrared surface-enhanced Raman spectroscopy: gold surfaces, 10-day stability, and improved accuracy. Anal. Chem. 77, 4013–4019 (2005).
Mahajan, S. et al. Tuning plasmons on nano-structured substrates for NIR-SERS. Phys. Chem. Chem. Phys. 9, 104–109 (2007).
Greeneltch, N. G. et al. Near-infrared surface-enhanced Raman spectroscopy (NIR-SERS) for the identification of eosin Y: theoretical calculations and evaluation of two different nanoplasmonic substrates. J. Phys. Chem. A 116, 11863–11869 (2012).
Yonzon, C. R., Haynes, C. L., Zhang, X., Walsh, J. T. & Van Duyne, R. P. A glucose biosensor based on surface-enhanced Raman scattering: improved partition layer, temporal stability, reversibility, and resistance to serum protein interference. Anal. Chem. 76, 78–85 (2004).
Asharani, P. V., Yi Lian, W., Zhiyuan, G. & Suresh, V. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19, 255102 (2008).
Asharani, P. V., Mun, G. L. K., Hande, M. P. & Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3, 279–290 (2009).
Liu, W. et al. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4, 319–330 (2010).
Shukla, R. et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21, 10644–10654 (2005).
Averitt, R. D., Sarkar, D. & Halas, N. J. Plasmon resonance shifts of Au-coated Au2S nanoshells: insight into multicomponent nanoparticle growth. Phys. Rev. Lett. 78, 4217–4220 (1997).
Oldenburg, S. J., Averitt, R. D., Westcott, S. L. & Halas, N. J. Nanoengineering of optical resonances. Chem. Phys. Lett. 288, 243–247 (1998).
Oldenburg, S. J., Westcott, S. L., Averitt, R. D. & Halas, N. J. Surface enhanced Raman scattering in the near infrared using metal nanoshell substrates. J. Chem. Phys. 111, 4729–4735 (1999).
Küstner, B. et al. SERS labels for red laser excitation: silica-encapsulated SAMs on tunable gold/silver nanoshells. Angew. Chem. Int. Ed. 48, 1950–1953 (2009).
Kang, H. et al. Near-infrared SERS nanoprobes with plasmonic Au/Ag hollow-shell assemblies for in vivo multiplex detection. Adv. Funct. Mater. 23, 3719–3727 (2013).
Sun, Y., Mayers, B. T. & Xia, Y. Template-engaged replacement reaction: a one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2, 481–485 (2002).
Liang, H. P., Wan, L. J., Bai, C. L. & Jiang, L. Gold hollow nanospheres: tunable surface plasmon resonance controlled by interior-cavity sizes. J. Phys. Chem. B 109, 7795–7800 (2005).
Schwartzberg, A. M., Olson, T. Y., Talley, C. E. & Zhang, J. Z. Synthesis, characterization, and tunable optical properties of hollow gold nanospheres. J. Phys. Chem. B 110, 19935–19944 (2006).
Xie, H. N., Larmour, I. A., Smith, W. E., Faulds, K. & Graham, D. Surface-enhanced Raman scattering investigation of hollow gold nanospheres. J. Phys. Chem. C 116, 8338–8342 (2012).
Xie, H. N. et al. Synthesis and NIR optical properties of hollow gold nanospheres with LSPR greater than one micrometer. Nanoscale 5, 765–771 (2013).
Kearns, H., Shand, N. C., Smith, W. E., Faulds, K. & Graham, D. 1064 nm SERS of NIR active hollow gold nanotags. Phys. Chem. Chem. Phys. 17, 1980–1986 (2015).
Adams, S. & Zhang, J. Z. Unique optical properties and applications of hollow gold nanospheres (HGNs). Coord. Chem. Rev. 320, 18–37 (2016).
von Maltzahn, G. et al. SERS-coded gold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv. Mater. 21, 3175–3180 (2009).
Wu, L. et al. A SERS-based immunoassay with highly increased sensitivity using gold/silver core–shell nanorods. Biosens. Bioelectron. 38, 94–99 (2012).
Zhang, Y., Qian, J., Wang, D., Wang, Y. & He, S. Multifunctional gold nanorods with ultrahigh stability and tunability for in vivo fluorescence imaging, SERS detection, and photodynamic therapy. Angew. Chem. Int. Ed. 52, 1148–1151 (2013).
Park, J.-H. et al. Cooperative nanoparticles for tumor detection and photothermally triggered drug delivery. Adv. Mater. 22, 880–885 (2010). The authors demonstrated that functionalized AuNRs, under thermal stimulus, can release a drug directly at tumour sites. The delivery was confirmed by SERS, highlighting the potential of this technique for diagnostic and therapeutic applications.
Harmsen, S. et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl. Med. 7, 271ra277 (2015).
Register, J. K. et al. In vivo detection of SERS-encoded plasmonic nanostars in human skin grafts and live animal models. Anal. Bioanal. Chem. 407, 8215–8224 (2015).
Zeng, L. et al. Raman reporter-coupled Agcore@Aushell nanostars for in vivo improved surface enhanced Raman scattering imaging and near-infrared-triggered photothermal therapy in breast cancers. ACS Appl. Mater. Interfaces 7, 16781–16791 (2015).
Liu, Y. et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 5, 946–960 (2015).
Maiti, K. K. et al. Multiplex cancer cell detection by SERS nanotags with cyanine and triphenylmethine Raman reporters. Chem. Commun. 47, 3514–3516 (2011).
Samanta, A., Das, R. K., Park, S. J., Maiti, K. K. & Chang, Y. T. Multiplexing SERS nanotags for the imaging of differentiated mouse embryonic stem cells (mESC) and detection of teratoma in vivo. Am. J. Nucl. Med. Mol. Imaging 4, 114–124 (2014).
Wustholz, K. L., Brosseau, C. L., Casadio, F. & Van Duyne, R. P. Surface-enhanced Raman spectroscopy of dyes: from single molecules to the artists' canvas. Phys. Chem. Chem. Phys. 11, 7350–7359 (2009).
McAughtrie, S., Lau, K., Faulds, K. & Graham, D. 3D optical imaging of multiple SERS nanotags in cells. Chem. Sci. 4, 3566–3572 (2013).
Bedics, M. A. et al. Extreme red shifted SERS nanotags. Chem. Sci. 6, 2302–2306 (2015).
Harmsen, S. et al. Rational design of a chalcogenopyrylium-based surface-enhanced resonance Raman scattering nanoprobe with attomolar sensitivity. Nat. Commun. 6, 9 (2015). A series of NIR dyes was designed and used with AuNPs to afford biocompatible SERRS probes that allow imaging at exceptionally high sensitivities.
Kearns, H. et al. Sensitive SERS nanotags for use with 1550 nm (retina-safe) laser excitation. Analyst 141, 5062–5065 (2016).
Dinish, U. S. et al. Single molecule with dual function on nanogold: biofunctionalized construct for in vivo photoacoustic imaging and SERS biosensing. Adv. Funct. Mater. 25, 2316–2325 (2015).
McQueenie, R. et al. Detection of inflammation in vivo by surface-enhanced Raman scattering provides higher sensitivity than conventional fluorescence imaging. Anal. Chem 84, 5968–5975 (2012).
Wu, X., Chen, J., Wu, M. & Zhao, J. X. Aptamers: active targeting ligands for cancer diagnosis and therapy. Theranostics 5, 322–344 (2015).
Zong, S. et al. A SERS and fluorescence dual mode cancer cell targeting probe based on silica coated Au@Ag core–shell nanorods. Talanta 97, 368–375 (2012).
Iacono, P., Karabeber, H. & Kircher, M. F. A ‘schizophotonic’ all-in-one nanoparticle coating for multiplexed SE(R)RS biomedical imaging. Angew. Chem. Int. Ed. 53, 11756–11761 (2014).
Liu, Z. M. et al. Dye-free near-infrared surface-enhanced Raman scattering nanoprobes for bioimaging and high-performance photothermal cancer therapy. Nanoscale 7, 6754–6761 (2015).
Jiang, C. H., Wang, Y., Wang, J. W., Song, W. & Lu, L. H. Achieving ultrasensitive in vivo detection of bone crack with polydopamine-capsulated surface-enhanced Raman nanoparticle. Biomaterials 114, 54–61 (2017).
Kim, J. H. et al. Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal. Chem. 78, 6967–6973 (2006).
Zhang, Z. et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 24, 1418–1423 (2012).
Doering, W. E. & Nie, S. Spectroscopic tags using dye-embedded nanoparticles and surface-enhanced Raman scattering. Anal. Chem. 75, 6171–6176 (2003).
Qian, X. et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 26, 83–90 (2008). The first application of a SERS probe for cancer detection in vivo. The probes comprised AuNPs functionalized with diethylthiatricarbocyanine, a protective PEG layer and single-chain variable fragment antibodies, to selectively bind EGFR.
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Potara, M., Baia, M., Farcau, C. & Astilean, S. Chitosan-coated anisotropic silver nanoparticles as a SERS substrate for single-molecule detection. Nanotechnology 23, 055501 (2012).
Potara, M. et al. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly sensitive plasmonic platforms for intracellular SERS sensing and imaging. Nanoscale 5, 6013–6022 (2013).
Keren, S. et al. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 5844–5849 (2008).
Bohndiek, S. E. et al. A small animal Raman instrument for rapid, wide-area, spectroscopic imaging. Proc. Natl Acad. Sci. USA 110, 12408–12413 (2013).
Zavaleta, C. L. et al. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc. Natl Acad. Sci. USA 110, E2288–E2297 (2013). This article describes the development of a fibre-based Raman device that can be inserted through a clinical endoscope and detect up to ten labelled nanosensors using SERS with excellent sensitivity.
Funovics, M. A., Alencar, H., Montet, X., Weissleder, R. & Mahmood, U. Simultaneous fluorescence imaging of protease expression and vascularity during murine colonoscopy for colonic lesion characterization. Gastrointest. Endosc. 64, 589–597 (2006).
Zavaleta, C. L. et al. Preclinical evaluation of Raman nanoparticle biodistribution for their potential use in clinical endoscopy imaging. Small 7, 2232–2240 (2011).
Thakor, A. S. et al. Oxidative stress mediates the effects of Raman-active gold nanoparticles in human cells. Small 7, 126–136 (2011).
Thakor, A. S. et al. The fate and toxicity of Raman-active silica–gold nanoparticles in mice. Sci. Transl. Med. 3, 79ra33 (2011).
Jeong, S. et al. Fluorescence–Raman dual modal endoscopic system for multiplexed molecular diagnostics. Sci. Rep. 5, 9455 (2015).
Wang, Y. W., Kang, S., Khan, A., Bao, P. Q. & Liu, J. T. C. In vivo multiplexed molecular imaging of esophageal cancer via spectral endoscopy of topically applied SERS nanoparticles. Biomed. Opt. Express 6, 3714–3723 (2015).
Garai, E. et al. A real-time clinical endoscopic system for intraluminal, multiplexed imaging of surface-enhanced Raman scattering nanoparticles. PLoS One 10, e0123185 (2015). A unique endoscopic opto-electromechanical Raman device was the first instrument to allow SERS imaging of tissue during gastrointestinal endoscopy.
Stone, N., Faulds, K., Graham, D. & Matousek, P. Prospects of deep Raman spectroscopy for noninvasive detection of conjugated surface enhanced resonance Raman scattering nanoparticles buried within 25 mm of mammalian tissue. Anal. Chem. 82, 3969–3973 (2010). The first report of SESORS.
Stone, N. et al. Surface enhanced spatially offset Raman spectroscopic (SESORS) imaging — the next dimension. Chem. Sci. 2, 776–780 (2011).
Xie, H. N. et al. Tracking bisphosphonates through a 20 mm thick porcine tissue by using surface-enhanced spatially offset Raman spectroscopy. Angew. Chem. Int. Ed. 51, 8509–8511 (2012).
Sharma, B., Ma, K., Glucksberg, M. R. & Van Duyne, R. P. Seeing through bone with surface-enhanced spatially offset Raman spectroscopy. J. Am. Chem. Soc. 135, 17290–17293 (2013).
Tonyushkina, K. & Nichols, J. H. Glucose meters: a review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 3, 971–980 (2009).
Lyandres, O. et al. Progress toward an in vivo surface-enhanced Raman spectroscopy glucose sensor. Diabetes Technol. Ther. 10, 257–265 (2008).
Shafer-Peltier, K. E., Haynes, C. L., Glucksberg, M. R. & Van Duyne, R. P. Toward a glucose biosensor based on surface-enhanced Raman scattering. J. Am. Chem. Soc. 125, 588–593 (2003).
Lyandres, O. et al. Real-time glucose sensing by surface-enhanced Raman spectroscopy in bovine plasma facilitated by a mixed decanethiol/mercaptohexanol partition layer. Anal. Chem. 77, 6134–6139 (2005).
Qi, G. et al. Glucose oxidase probe as a surface-enhanced Raman scattering sensor for glucose. Anal. Bioanal. Chem. 408, 7513–7520 (2016).
Maiti, K. K. et al. Development of biocompatible SERS nanotag with increased stability by chemisorption of reporter molecule for in vivo cancer detection. Biosens. Bioelectron 26, 398–403 (2010).
Maiti, K. K. et al. Multiplex targeted in vivo cancer detection using sensitive near-infrared SERS nanotags. Nano Today 7, 85–93 (2012).
Mallia, R. J., McVeigh, P. Z., Fisher, C. J., Veilleux, I. & Wilson, B. C. Wide-field multiplexed imaging of EGFR-targeted cancers using topical application of NIR SERS nanoprobes. Nanomedicine 10, 89–101 (2015).
Sinha, L. et al. Quantification of the binding potential of cell-surface receptors in fresh excised specimens via dual-probe modeling of SERS nanoparticles. Sci. Rep. 5, 8582 (2015).
Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 18, 829–834 (2012). The authors report the design of a nanoprobe that can be imaged using three different techniques. The advantages of these complementary methods enabled successful brain tumour resection.
Sershen, S. R., Westcott, S. L., Halas, N. J. & West, J. L. Temperature-sensitive polymer–nanoshell composites for photothermally modulated drug delivery. J. Biomed. Mater. Res. 51, 293–298 (2000).
Huschka, R. et al. Light-induced release of DNA from gold nanoparticles: nanoshells and nanorods. J. Am. Chem. Soc. 133, 12247–12255 (2011).
Huo, S. et al. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 8, 5852–5862 (2014).
Mackanic, D. G., Mabbott, S., Faulds, K. & Graham, D. Analysis of photothermal release of oligonucleotides from hollow gold nanospheres by surface-enhanced Raman scattering. J. Phys. Chem. C 120, 20677–20683 (2016).
Cheng, Y. et al. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 130, 10643–10647 (2008).
Xia, Y. et al. Three dimensional plasmonic assemblies of AuNPs with an overall size of sub-200 nm for chemo-photothermal synergistic therapy of breast cancer. Nanoscale 8, 18682–18692 (2016).
Kang, B., Afifi, M. M., Austin, L. A. & El-Sayed, M. A. Exploiting the nanoparticle plasmon effect: observing drug delivery dynamics in single cells via Raman/fluorescence imaging spectroscopy. ACS Nano 7, 7420–7427 (2013).
Gao, Y. et al. Multifunctional gold nanostar-based nanocomposite: synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials 60, 31–41 (2015).
Acknowledgements
K.F. and S.L. thank the Leverhulme Trust for financial support through Research Project Grant RPG-2012-758.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Laing, S., Jamieson, L., Faulds, K. et al. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat Rev Chem 1, 0060 (2017). https://doi.org/10.1038/s41570-017-0060
Published:
DOI: https://doi.org/10.1038/s41570-017-0060
This article is cited by
-
Deep learning for tumor margin identification in electromagnetic imaging
Scientific Reports (2023)
-
Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning
Nature Communications (2023)
-
Molecular imaging: design mechanism and bioapplications
Science China Chemistry (2023)
-
Principles, Methods, and Real-Time Applications of Bacteriophage-Based Pathogen Detection
Molecular Biotechnology (2023)
-
Near-infrared II plasmonic porous cubic nanoshells for in vivo noninvasive SERS visualization of sub-millimeter microtumors
Nature Communications (2022)