Abstract
Cardiogenesis is a complex developmental process involving multiple overlapping stages of cell fate specification, proliferation, differentiation, and morphogenesis. Precise spatiotemporal coordination between the different cardiogenic processes is ensured by intercellular signalling crosstalk and tissue–tissue interactions. Notch is an intercellular signalling pathway crucial for cell fate decisions during multicellular organismal development and is aptly positioned to coordinate the complex signalling crosstalk required for progressive cell lineage restriction during cardiogenesis. In this Review, we describe the role of Notch signalling and the crosstalk with other signalling pathways during the differentiation and patterning of the different cardiac tissues and in cardiac valve and ventricular chamber development. We examine how perturbation of Notch signalling activity is linked to congenital heart diseases affecting the neonate and adult, and discuss studies that shed light on the role of Notch signalling in heart regeneration and repair after injury.
Key points
-
Vertebrate heart development is a complex multistep process that relies on the contribution of several cellular lineages in a spatiotemporally regulated manner.
-
Notch is a highly conserved, local cell–cell signalling pathway required for proliferation, differentiation, and tissue patterning in a variety of tissues, including the heart.
-
Notch signalling in the endocardium regulates cardiac specification, progenitor cell differentiation, valve primordium formation and morphogenesis, ventricular trabeculation and compaction, and coronary vessel development.
-
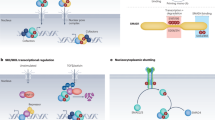
Notch coordinates cellular interactions during heart development by cross talking with other fundamental signalling pathways, including WNT, bone morphogenetic protein, and neuregulin 1–ERBB.
-
Defective Notch signalling during heart development causes congenital heart disease affecting neonates and adults.
-
Notch regulates cardiac regenerative processes in zebrafish, providing an incentive for evaluating Notch-based cell therapies in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




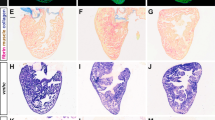
Immunofluorescence images adapted from ref.38, Springer Nature Limited.

Similar content being viewed by others
References
Garg, V. et al. Mutations in NOTCH1 cause aortic valve disease. Nature 437, 270–274 (2005).
Luxan, G. et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 19, 193–201 (2013).
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 16, 243–251 (1997).
Oda, T. et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet. 16, 235–242 (1997).
Abu-Issa, R. & Kirby, M. L. Heart field: from mesoderm to heart tube. Annu. Rev. Cell Dev. Biol. 23, 45–68 (2007).
Evans, S. M., Yelon, D., Conlon, F. L. & Kirby, M. L. Myocardial lineage development. Circ. Res. 107, 1428–1444 (2010).
Noseda, M., Peterkin, T., Simoes, F. C., Patient, R. & Schneider, M. D. Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ. Res. 108, 129–152 (2011).
Vincent, S. D. & Buckingham, M. E. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr. Top. Dev. Biol. 90, 1–41 (2010).
Saga, Y. et al. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122, 2769–2778 (1996).
Lescroart, F. et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359, 1177–1181 (2018).
Rones, M. S., McLaughlin, K. A., Raffin, M. & Mercola, M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127, 3865–3876 (2000).
Contakos, S. P., Gaydos, C. M., Pfeil, E. C. & McLaughlin, K. A. Subdividing the embryo: a role for Notch signaling during germ layer patterning in Xenopus laevis. Dev. Biol. 288, 294–307 (2005).
Miazga, C. M. & McLaughlin, K. A. Coordinating the timing of cardiac precursor development during gastrulation: a new role for Notch signaling. Dev. Biol. 333, 285–296 (2009).
Schroeder, T. et al. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc. Natl Acad. Sci. USA 100, 4018–4023 (2003).
Nemir, M., Croquelois, A., Pedrazzini, T. & Radtke, F. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ. Res. 98, 1471–1478 (2006).
Schroeder, T. et al. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech. Dev. 123, 570–579 (2006).
Lowell, S., Benchoua, A., Heavey, B. & Smith, A. G. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLOS Biol. 4, e121 (2006).
Chen, V. C., Stull, R., Joo, D., Cheng, X. & Keller, G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat. Biotechnol. 26, 1169–1178 (2008).
Bray, S. J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 17, 722–735 (2016).
Oka, C. et al. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 121, 3291–3301 (1995).
Souilhol, C., Cormier, S., Tanigaki, K., Babinet, C. & Cohen-Tannoudji, M. RBP-Jkappa-dependent notch signaling is dispensable for mouse early embryonic development. Mol. Cell. Biol. 26, 4769–4774 (2006).
Timmerman, L. A. et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 18, 99–115 (2004).
Munger, T. M. et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 87, 866–873 (1993).
de la Pompa, J. L. & Epstein, J. A. Coordinating tissue interactions: notch signaling in cardiac development and disease. Dev. Cell. 22, 244–254 (2012).
Munshi, N. V. Gene regulatory networks in cardiac conduction system development. Circ. Res. 110, 1525–1537 (2012).
Yamada, M., Revelli, J. P., Eichele, G., Barron, M. & Schwartz, R. J. Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev. Biol. 228, 95–105 (2000).
Christoffels, V. M. et al. T-Box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev. Dyn. 229, 763–770 (2004).
Singh, M. K. et al. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development 132, 2697–2707 (2005).
Stennard, F. A. et al. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development 132, 2451–2462 (2005).
Kokubo, H., Tomita-Miyagawa, S., Hamada, Y. & Saga, Y. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development 134, 747–755 (2007).
Rutenberg, J. B. et al. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development 133, 4381–4390 (2006).
Ma, L., Lu, M. F., Schwartz, R. J. & Martin, J. F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132, 5601–5611 (2005).
Aanhaanen, W. T. et al. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ. Res. 104, 1267–1274 (2009).
Harrelson, Z. et al. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 131, 5041–5052 (2004).
Rivera-Feliciano, J. & Tabin, C. J. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev. Biol. 295, 580–588 (2006).
Papoutsi, T., Luna-Zurita, L., Prados, B., Zaffran, S. & de la Pompa, J. L. Bmp2 and Notch cooperate to pattern the embryonic endocardium. Development 145, dev163378 (2018).
Luna-Zurita, L. et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Invest. 120, 3493–3507 (2010).
D’Amato, G. et al. Sequential Notch activation regulates ventricular chamber development. Nat. Cell Biol. 18, 7–20 (2016).
Del Monte, G., Grego-Bessa, J., Gonzalez-Rajal, A., Bolos, V. & De La Pompa, J. L. Monitoring Notch1 activity in development: evidence for a feedback regulatory loop. Dev. Dyn. 236, 2594–2614 (2007).
Grego-Bessa, J. et al. Notch signaling is essential for ventricular chamber development. Dev. Cell. 12, 415–429 (2007).
Fischer, A. et al. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ. Res. 100, 856–863 (2007).
Watanabe, Y. et al. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development 133, 1625–1634 (2006).
Kisanuki, Y. Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
Rentschler, S. et al. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Invest. 121, 525–533 (2011).
Gillers, B. S. et al. Canonical wnt signaling regulates atrioventricular junction programming and electrophysiological properties. Circ. Res. 116, 398–406 (2015).
Dyer, L. A. & Kirby, M. L. The role of secondary heart field in cardiac development. Dev. Biol. 336, 137–144 (2009).
Kelly, R. G. The second heart field. Curr. Top. Dev. Biol. 100, 33–65 (2012).
Meilhac, S. M., Lescroart, F., Blanpain, C. & Buckingham, M. E. Cardiac cell lineages that form the heart. Cold Spring Harb Perspect Med. 4, a013888 (2014).
Ma, Q., Zhou, B. & Pu, W. T. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev. Biol. 323, 98–104 (2008).
Verzi, M. P., McCulley, D. J., De Val, S., Dodou, E. & Black, B. L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 287, 134–145 (2005).
Zaffran, S., Kelly, R. G., Meilhac, S. M., Buckingham, M. E. & Brown, N. A. Right ventricular myocardium derives from the anterior heart field. Circ. Res. 95, 261–268 (2004).
Rochais, F., Mesbah, K. & Kelly, R. G. Signaling pathways controlling second heart field development. Circ. Res. 104, 933–942 (2009).
Kwon, C. et al. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 11, 951–957 (2009).
Klaus, A. et al. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc. Natl Acad. Sci. USA 109, 10921–10926 (2012).
McDaniell, R. et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 79, 169–173 (2006).
McElhinney, D. B. et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 106, 2567–2574 (2002).
Eldadah, Z. A. et al. Familial tetralogy of Fallot caused by mutation in the jagged1 gene. Hum. Mol. Genet. 10, 163–169 (2001).
Greenway, S. C. et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat. Genet. 41, 931–935 (2009).
Neeb, Z., Lajiness, J. D., Bolanis, E. & Conway, S. J. Cardiac outflow tract anomalies. Wiley Interdiscip Rev. Dev. Biol. 2, 499–530 (2013).
Nakajima, M., Moriizumi, E., Koseki, H. & Shirasawa, T. Presenilin 1 is essential for cardiac morphogenesis. Dev. Dyn. 230, 795–799 (2004).
McCright, B., Lozier, J. & Gridley, T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129, 1075–1082 (2002).
High, F. A. et al. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J. Clin. Invest. 119, 1986–1996 (2009).
Ilagan, R. et al. Fgf8 is required for anterior heart field development. Development 133, 2435–2445 (2006).
Rochais, F. et al. Hes1 is expressed in the second heart field and is required for outflow tract development. PLOS One. 4, e6267 (2009).
Donovan, J., Kordylewska, A., Jan, Y. N. & Utset, M. F. Tetralogy of fallot and other congenital heart defects in Hey2 mutant mice. Curr. Biol. 12, 1605–1610 (2002).
Fischer, A., Schumacher, N., Maier, M., Sendtner, M. & Gessler, M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 18, 901–911 (2004).
Gessler, M. et al. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2 -/- mice. Curr. Biol. 12, 1601–1604 (2002).
Kokubo, H., Miyagawa-Tomita, S., Nakazawa, M., Saga, Y. & Johnson, R. L. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev. Biol. 278, 301–309 (2005).
Sakata, Y. et al. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc. Natl Acad. Sci. USA 99, 16197–16202 (2002).
Sakata, Y. et al. The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J. Mol. Cell Cardiol. 40, 267–273 (2006).
Shirai, M., Imanaka-Yoshida, K., Schneider, M. D., Schwartz, R. J. & Morisaki, T. T-Box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgfbeta2 expression and endocardial cushion formation. Proc. Natl Acad. Sci. USA 106, 18604–18609 (2009).
Shelton, E. L. & Yutzey, K. E. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev. Biol. 302, 376–388 (2007).
Armstrong, E. J. & Bischoff, J. Heart valve development: endothelial cell signaling and differentiation. Circ. Res. 95, 459–470 (2004).
MacGrogan, D. et al. Sequential ligand-dependent notch signaling activation regulates valve primordium formation and morphogenesis. Circ. Res. 118, 1480–1497 (2016).
Niessen, K. et al. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J. Cell Biol. 182, 315–325 (2008).
Wang, Y. et al. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLOS One. 8, e60244 (2013).
Samsa, L. A. et al. Cardiac contraction activates endocardial Notch signaling to modulate chamber maturation in zebrafish. Development 142, 4080–4091 (2015).
Walsh, E. C. & Stainier, D. Y. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science 293, 1670–1673 (2001).
Heidersbach, A. et al. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife 2, e01323 (2013).
Vermot, J. et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLOS Biol. 7, e1000246 (2009).
Steed, E. et al. klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nat. Commun. 7, 11646 (2016).
Donat, S. et al. Heg1 and Ccm1/2 proteins control endocardial mechanosensitivity during zebrafish valvulogenesis. Elife 7, e28939 (2018).
Pestel, J. et al. Real-time 3D visualization of cellular rearrangements during cardiac valve formation. Development 143, 2217–2227 (2016).
Beis, D. et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132, 4193–4204 (2005).
Gridley, T. Notch signaling in the vasculature. Curr. Top. Dev. Biol. 92, 277–309 (2010).
Hurlstone, A. F. et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425, 633–637 (2003).
Verhoeven, M. C., Haase, C., Christoffels, V. M., Weidinger, G. & Bakkers, J. Wnt signaling regulates atrioventricular canal formation upstream of BMP and Tbx2. Birth Defects Res. A Clin. Mol. Teratol. 91, 435–440 (2011).
Goddard, L. M. et al. Hemodynamic forces sculpt developing heart valves through a KLF2-WNT9B paracrine signaling axis. Dev. Cell. 43, 274–289.e5 (2017).
Plein, A., Fantin, A. & Ruhrberg, C. Neural crest cells in cardiovascular development. Curr. Top. Dev. Biol. 111, 183–200 (2015).
Phillips, H. M. et al. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 99, 452–460 (2013).
High, F. & Epstein, J. A. Signalling pathways regulating cardiac neural crest migration and differentiation. Novartis Found. Symp. 283, 152–161; discussion 161–154, 238–141 (2007).
MacGrogan, D. et al. How to make a heart valve: from embryonic development to bioengineering of living valve substitutes. Cold Spring Harb Perspect Med. 4, a013912 (2014).
Lin, C. Y. et al. The secondary heart field is a new site of calcineurin/Nfatc1 signaling for semilunar valve development. J. Mol. Cell Cardiol. 52, 1096–1102 (2012).
Jain, R. et al. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J. Clin. Invest. 121, 422–430 (2011).
Wang, Y. et al. Notch-Tnf signalling is required for development and homeostasis of arterial valves. Eur. Heart J. 38, 675–686 (2017).
Foffa, I. et al. Sequencing of NOTCH1, GATA5, TGFBR1 and TGFBR2 genes in familial cases of bicuspid aortic valve. BMC Med. Genet. 14, 44 (2013).
McBride, K. L. et al. NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum. Mol. Genet. 17, 2886–2893 (2008).
McKellar, S. H. et al. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J. Thorac Cardiovasc. Surg. 134, 290–296 (2007).
Mohamed, S. A. et al. Novel missense mutations (p. T596M and p. P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem. Biophys. Res. Commun. 345, 1460–1465 (2006).
Kerstjens-Frederikse, W. S. et al. Cardiovascular malformations caused by NOTCH1 mutations do not keep left: data on 428 probands with left-sided CHD and their families. Genet. Med. 18, 914–923 (2016).
Nigam, V. & Srivastava, D. Notch1 represses osteogenic pathways in aortic valve cells. J. Mol. Cell Cardiol. 47, 828–834 (2009).
Nus, M. et al. Diet-induced aortic valve disease in mice haploinsufficient for the notch pathway effector RBPJK/CSL. Arterioscler Thromb. Vasc. Biol. 31, 1580–1588 (2011).
Theodoris, C. V. et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell 160, 1072–1086 (2015).
Prakash, S. K. et al. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the International BAVCon (Bicuspid Aortic Valve Consortium). J. Am. Coll. Cardiol. 64, 832–839 (2014).
Sedmera, D., Pexieder, T., Hu, N. & Clark, E. B. Developmental changes in the myocardial architecture of the chick. Anat. Rec. 248, 421–432 (1997).
Sedmera, D., Pexieder, T., Vuillemin, M., Thompson, R. P. & Anderson, R. H. Developmental patterning of the myocardium. Anat. Rec. 258, 319–337 (2000).
Hu, N., Sedmera, D., Yost, H. J. & Clark, E. B. Structure and function of the developing zebrafish heart. Anat. Rec. 260, 148–157 (2000).
Sedmera, D. et al. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 274, 773–777 (2003).
Park, D. S. et al. Pocket proteins critically regulate cell cycle exit of the trabecular myocardium and the ventricular conduction system. Biol. Open. 2, 968–978 (2013).
Rentschler, S. et al. Visualization and functional characterization of the developing murine cardiac conduction system. Development 128, 1785–1792 (2001).
Moorman, A. F. & Christoffels, V. M. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 83, 1223–1267 (2003).
Christoffels, V. M. & Moorman, A. F. Development of the cardiac conduction system: why are some regions of the heart more arrhythmogenic than others? Circ. Arrhythm Electrophysiol. 2, 195–207 (2009).
Jimenez-Amilburu, V. et al. In vivo visualization of cardiomyocyte apicobasal polarity reveals epithelial to mesenchymal-like transition during cardiac trabeculation. Cell Rep. 17, 2687–2699 (2016).
Li, J. et al. Single-cell lineage tracing reveals that oriented cell division contributes to trabecular morphogenesis and regional specification. Cell Rep. 15, 158–170 (2016).
Passer, D., van de Vrugt, A., Atmanli, A. & Domian, I. J. Atypical protein kinase C-dependent polarized cell division is required for myocardial trabeculation. Cell Rep. 14, 1662–1672 (2016).
Le Garrec, J. F. et al. Quantitative analysis of polarity in 3D reveals local cell coordination in the embryonic mouse heart. Development 140, 395–404 (2013).
Staudt, D. W. et al. High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 141, 585–593 (2014).
de Luxan, G., D’Amato, G., MacGrogan, D. & de la Pompa, J. L. Endocardial Notch signaling in cardiac development and disease. Circ. Res. 118, e1–e18 (2015).
Chen, H. et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131, 2219–2231 (2004).
Gerety, S. S. & Anderson, D. J. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 129, 1397–1410 (2002).
Meyer, D. & Birchmeier, C. Multiple essential functions of neuregulin in development. Nature 378, 386–390 (1995).
VanDusen, N. J. et al. Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell Rep. 9, 2071–2083 (2014).
Bjarnadottir, T. K., Fredriksson, R. & Schioth, H. B. The adhesion GPCRs: a unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell. Mol. Life Sci. 64, 2104–2119 (2007).
Waller-Evans, H. et al. The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLOS One. 5, e14047 (2010).
Patra, C. et al. Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc. Natl Acad. Sci. USA 110, 16898–16903 (2013).
Del Monte-Nieto, G. et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 557, 439–445 (2018).
Tian, X. et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 8, 87 (2017).
Tian, X. et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science 345, 90–94 (2014).
Del Monte, G. et al. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ. Res. 108, 824–836 (2011).
Jenni, R., Oechslin, E., Schneider, J., Attenhofer Jost, C. & Kaufmann, P. A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86, 666–671 (2001).
Red-Horse, K., Ueno, H., Weissman, I. L. & Krasnow, M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010).
Perez-Pomares, J. M. et al. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 46, 1005–1013 (2002).
Tian, X. et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 23, 1075–1090 (2013).
Wu, B. et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151, 1083–1096 (2012).
Zhang, H. et al. Genetic lineage tracing identifies endocardial origin of liver vasculature. Nat. Genet. 48, 537–543 (2016).
Sharma, B. et al. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and Apj-deficient hearts. Dev. Cell. 42, 655–666.e3 (2017).
Cano, E. et al. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proc. Natl Acad. Sci. USA 113, 656–661 (2016).
Katz, T. C. et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell. 22, 639–650 (2012).
Zhou, B. & Pu, W. T. Genetic Cre-loxP assessment of epicardial cell fate using Wt1-driven Cre alleles. Circ. Res. 111, e276–e280 (2012).
Riley, P. R. An epicardial floor plan for building and rebuilding the mammalian heart. Curr. Top. Dev. Biol. 100, 233–251 (2012).
Grieskamp, T., Rudat, C., Ludtke, T. H., Norden, J. & Kispert, A. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ. Res. 108, 813–823 (2011).
Munch, J., Gonzalez-Rajal, A. & de la Pompa, J. L. Notch regulates blastema proliferation and prevents differentiation during adult zebrafish fin regeneration. Development 140, 1402–1411 (2013).
de Oliveira-Carlos, V., Ganz, J., Hans, S., Kaslin, J. & Brand, M. Notch receptor expression in neurogenic regions of the adult zebrafish brain. PLOS One. 8, e73384 (2013).
Grotek, B., Wehner, D. & Weidinger, G. Notch signaling coordinates cellular proliferation with differentiation during zebrafish fin regeneration. Development 140, 1412–1423 (2013).
Beck, C. W., Christen, B. & Slack, J. M. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev. Cell. 5, 429–439 (2003).
Dias, T. B., Yang, Y. J., Ogai, K., Becker, T. & Becker, C. G. Notch signaling controls generation of motor neurons in the lesioned spinal cord of adult zebrafish. J. Neurosci. 32, 3245–3252 (2012).
Wan, J., Ramachandran, R. & Goldman, D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev. Cell. 22, 334–347 (2012).
Zhang, R. et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 498, 497–501 (2013).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Gonzalez-Rosa, J. M., Martin, V., Peralta, M., Torres, M. & Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138, 1663–1674 (2011).
Schnabel, K., Wu, C. C., Kurth, T. & Weidinger, G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLOS One. 6, e18503 (2011).
Chablais, F., Veit, J., Rainer, G. & Jazwinska, A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 11, 21 (2011).
Jopling, C. et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 (2010).
Lepilina, A. et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619 (2006).
Raya, A. et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl Acad. Sci. USA 100 (Suppl. 1), 11889–11895 (2003).
Munch, J., Grivas, D., Gonzalez-Rajal, A., Torregrosa-Carrion, R. & de la Pompa, J. L. Notch signalling restricts inflammation and serpine1 expression in the dynamic endocardium of the regenerating zebrafish heart. Development 144, 1425–1440 (2017).
Zhao, L. et al. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 111, 1403–1408 (2014).
Hellstrom, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Suchting, S. et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl Acad. Sci. USA 104, 3225–3230 (2007).
Darehzereshki, A. et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 399, 91–99 (2015).
Adler, E. D. et al. The cardiomyocyte lineage is critical for optimization of stem cell therapy in a mouse model of myocardial infarction. FASEB J. 24, 1073–1081 (2010).
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464, 601–605 (2010).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Collesi, C., Zentilin, L., Sinagra, G. & Giacca, M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J. Cell Biol. 183, 117–128 (2008).
Campa, V. M. et al. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J. Cell Biol. 183, 129–141 (2008).
Gude, N. A. et al. Activation of Notch-mediated protective signaling in the myocardium. Circ. Res. 102, 1025–1035 (2008).
Kratsios, P. et al. Distinct roles for cell-autonomous Notch signaling in cardiomyocytes of the embryonic and adult heart. Circ. Res. 106, 559–572 (2010).
Blaumueller, C. M., Qi, H., Zagouras, P. & Artavanis-Tsakonas, S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90, 281–291 (1997).
Logeat, F. et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl Acad. Sci. USA 95, 8108–8112 (1998).
Rand, M. D. et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20, 1825–1835 (2000).
Bettenhausen, B., Hrabe de Angelis, M., Simon, D., Guenet, J. L. & Gossler, A. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development 121, 2407–2418 (1995).
Dunwoodie, S. L., Henrique, D., Harrison, S. M. & Beddington, R. S. Mouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development 124, 3065–3076 (1997).
Shutter, J. R. et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 14, 1313–1318 (2000).
Lindsell, C. E., Shawber, C. J., Boulter, J. & Weinmaster, G. Jagged: a mammalian ligand that activates Notch1. Cell 80, 909–917 (1995).
Shawber, C., Boulter, J., Lindsell, C. E. & Weinmaster, G. Jagged2: a serrate-like gene expressed during rat embryogenesis. Dev. Biol. 180, 370–376 (1996).
Chillakuri, C. R. et al. Structural analysis uncovers lipid-binding properties of Notch ligands. Cell Rep. 5, 861–867 (2013).
Panin, V. M., Papayannopoulos, V., Wilson, R. & Irvine, K. D. Fringe modulates Notch-ligand interactions. Nature 387, 908–912 (1997).
Yang, L. T. et al. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol. Biol. Cell. 16, 927–942 (2005).
Itoh, M. et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 4, 67–82 (2003).
Musse, A. A., Meloty-Kapella, L. & Weinmaster, G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin. Cell Dev. Biol. 23, 429–436 (2012).
Jarriault, S. et al. Signalling downstream of activated mammalian Notch. Nature. 377, 355–358 (1995).
Borggrefe, T. & Oswald, F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 66, 1631–1646 (2009).
Kovall, R. A. & Blacklow, S. C. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr. Top. Dev. Biol. 92, 31–71 (2010).
Fischer, A. & Gessler, M. Delta-Notch — and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 35, 4583–4596 (2007).
Guruharsha, K. G., Kankel, M. W. & Artavanis-Tsakonas, S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 (2012).
Siu, S. C. & Silversides, C. K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 55, 2789–2800 (2010).
Fernandez, B. et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J. Am. Coll. Cardiol. 54, 2312–2318 (2009).
Aboulhosn, J. & Child, J. S. Left ventricular outflow obstruction: subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation 114, 2412–2422 (2006).
Michelena, H. I. et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 129, 2691–2704 (2014).
Tadros, T. M., Klein, M. D. & Shapira, O. M. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation 119, 880–890 (2009).
Cripe, L., Andelfinger, G., Martin, L. J., Shooner, K. & Benson, D. W. Bicuspid aortic valve is heritable. J. Am. Coll. Cardiol. 44, 138–143 (2004).
Hinton, R. B. et al. Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve. J. Am. Coll. Cardiol. 53, 1065–1071 (2009).
Martin, L. J. et al. Evidence in favor of linkage to human chromosomal regions 18q, 5q and 13q for bicuspid aortic valve and associated cardiovascular malformations. Hum. Genet. 121, 275–284 (2007).
Jenni, R., Oechslin, E. N. & van der Loo, B. Isolated ventricular non-compaction of the myocardium in adults. Heart 93, 11–15 (2007).
Oechslin, E. & Jenni, R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur. Heart J. 32, 1446–1456 (2011).
Chin, T. K., Perloff, J. K., Williams, R. G., Jue, K. & Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82, 507–513 (1990).
Maron, B. J. et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113, 1807–1816 (2006).
Towbin, J. A., Lorts, A. & Jefferies, J. L. Left ventricular non-compaction cardiomyopathy. Lancet 386, 813–825 (2015).
Ritter, M. et al. Isolated noncompaction of the myocardium in adults. Mayo Clin. Proc. 72, 26–31 (1997).
Oechslin, E. N., Attenhofer Jost, C. H., Rojas, J. R., Kaufmann, P. A. & Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 36, 493–500 (2000).
Stollberger, C. & Finsterer, J. Value of cardiac magnetic resonance imaging in the diagnosis of left ventricular hypertrabeculation/noncompaction. J. Cardiovasc. Magn. Reson. 6, 959–960; author reply 961–962 (2004).
Petersen, S. E. et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 46, 101–105 (2005).
Captur, G. & Nihoyannopoulos, P. Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int. J. Cardiol. 140, 145–153 (2010).
Klaassen, S. et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 117, 2893–2901 (2008).
Postma, A. V. et al. Mutations in the sarcomere gene MYH7 in Ebstein anomaly. Circ. Cardiovasc. Genet. 4, 43–50 (2011).
Ichida, F. et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 103, 1256–1263 (2001).
Shan, L. et al. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol. Genet. Metab. 93, 468–474 (2008).
Hermida-Prieto, M. et al. Familial dilated cardiomyopathy and isolated left ventricular noncompaction associated with lamin A/C gene mutations. Am. J. Cardiol. 94, 50–54 (2004).
Acknowledgements
The authors thank present and past members of the laboratory for their contribution to this Review and apologize for the omission of studies not discussed or cited because of space limitations. J.L.d.l.P. is funded by grants SAF2016-78370-R, CB16/11/00399 (CIBER CV), and RD16/0011/0021 (TERCEL) from the Ministerio de Ciencia, Innovación y Universidades and grants from the Fundación BBVA (Ref. BIO14_298) and Fundación La Marató (Ref. 20153431). J.L.d.l.P.'s work was supported in part with funds from the ERDF. CNIC is supported by the Ministerio de Ciencia, Innovación y Universidades and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Author information
Authors and Affiliations
Contributions
D.M. and J.M. researched data for the article. D.M and J.L.d.l.P. provided substantial contribution to the discussion of the content. All the authors wrote the article, and J.L.d.l.P. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
MacGrogan, D., Münch, J. & de la Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol 15, 685–704 (2018). https://doi.org/10.1038/s41569-018-0100-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0100-2
This article is cited by
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy
Cell Communication and Signaling (2024)
-
HAND factors regulate cardiac lineage commitment and differentiation from human pluripotent stem cells
Stem Cell Research & Therapy (2024)
-
Canonical notch activation in patients with scrub typhus: association with organ dysfunction and poor outcome
Infection (2024)
-
HES1-mediated down-regulation of miR-138 sustains NOTCH1 activation and promotes proliferation and invasion in renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Comparative genomics analyses reveal sequence determinants underlying interspecies variations in injury-responsive enhancers
BMC Genomics (2023)