Abstract
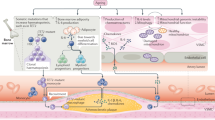
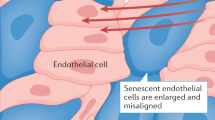
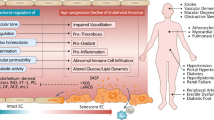
Ageing is the main risk factor for the development of cardiovascular diseases. A central mechanism by which ageing promotes vascular pathologies is compromising endothelial health. The age-related attenuation of endothelium-dependent dilator responses (endothelial dysfunction) associated with impairment of angiogenic processes and the subsequent pathological remodelling of the microcirculation contribute to compromised tissue perfusion and exacerbate functional decline in older individuals. This Review focuses on cellular, molecular, and functional changes that occur in the endothelium during ageing. We explore the links between oxidative and nitrative stress and the conserved molecular pathways affecting endothelial dysfunction and impaired angiogenesis during ageing. We also speculate on how these pathological processes could be therapeutically targeted. An improved understanding of endothelial biology in older patients is crucial for all cardiologists because maintenance of a competently functioning endothelium is critical for adequate tissue perfusion and long-term cardiac health.
Key points
-
Age-related endothelial dysfunction associated with impairment of angiogenic processes and the subsequent pathological remodelling of the microcirculation contribute to compromised tissue perfusion and exacerbate functional decline in older individuals.
-
The mechanisms underlying age-related endothelial dysfunction are multifaceted and are likely to involve increased oxidative and nitrative stress and alterations in the conserved molecular pathways affecting common ageing processes.
-
Age-related impairment of angiogenesis probably results from reduced nitric oxide bioavailability, metabolic dysregulation, altered angiomiR expression, NRF2 dysfunction, endothelial senescence and apoptosis, alterations in anti-geronic circulating factors and inducers of angiogenesis, and impaired pericyte function.
-
Anti-ageing interventions that prevent or reverse age-related endothelial dysfunction and improve angiogenesis are expected to confer cardiovascular protection and delay functional decline in older individuals, extending health span.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ungvari, Z., Kaley, G., de Cabo, R., Sonntag, W. E. & Csiszar, A. Mechanisms of vascular aging: new perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1028–1041 (2010).
Lerman, A. & Zeiher, A. M. Endothelial function: cardiac events. Circulation 111, 363–368 (2005).
Asai, K. et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler. Thromb. Vasc. Biol. 20, 1493–1499 (2000).
Csiszar, A. et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 90, 1159–1166 (2002).
Donato, A. J. et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 589, 4545–4554 (2011).
Li, W. et al. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J. Mol. Cell. Cardiol. 37, 671–680 (2004).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Jablonski, K. L., Seals, D. R., Eskurza, I., Monahan, K. D. & Donato, A. J. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J. Appl. Physiol. 103, 1715–1721 (2007).
Donato, A. J. et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ. Res. 100, 1659–1666 (2007).
Adler, A. et al. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am. J. Physiol. Heart Circ. Physiol. 285, H1015–H1022 (2003).
Sun, D. et al. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am. J. Physiol. Heart Circ. Physiol. 286, H2249–H2256 (2004).
Francia, P. et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110, 2889–2895 (2004).
Tschudi, M. R. et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J. Clin. Invest. 98, 899–905 (1996).
Tanabe, T. et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol. Scand. 178, 3–10 (2003).
Woodman, C. R., Price, E. M. & Laughlin, M. H. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J. Appl. Physiol. 93, 1685–1690 (2002).
Matsushita, H. et al. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ. Res. 89, 793–798 (2001).
Hoffmann, J. et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ. Res. 89, 709–715 (2001).
Sindler, A. L., Delp, M. D., Reyes, R., Wu, G. & Muller-Delp, J. M. Effects of aging and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J. Physiol. 587, 3885–3897 (2009).
Berkowitz, D. E. et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108, 2000–2006 (2003).
Csiszar, A. et al. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am. J. Pathol. 170, 388–398 (2007).
Flavahan, S., Chang, F. & Flavahan, N. A. Local renin-angiotensin system mediates endothelial dilator dysfunction in aging arteries. Am. J. Physiol. Heart Circ. Physiol. 311, H849–H854 (2016).
Dai, D. F., Rabinovitch, P. S. & Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 110, 1109–1124 (2012).
Tarantini, S. et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17, e12731 (2018).
Gioscia-Ryan, R. A. et al. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 592, 2549–2561 (2014).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Wenzel, P. et al. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res. 80, 280–289 (2008).
Oelze, M. et al. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension 63, 390–396 (2014).
Ungvari, Z. I. et al. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 294, H2121–H2128 (2008).
van der Loo, B. et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 192, 1731–1744 (2000).
Csiszar, A. et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech. Ageing Dev. 130, 518–527 (2009).
Burns, E. M., Kruckeberg, T. W., Comerford, L. E. & Buschmann, M. T. Thinning of capillary walls and declining numbers of endothelial mitochondria in the cerebral cortex of the aging primate, Macaca nemestrina. J. Gerontol. 34, 642–650 (1979).
Burns, E. M., Kruckeberg, T. W. & Gaetano, P. K. Changes with age in cerebral capillary morphology. Neurobiol. Aging 2, 283–291 (1981).
Tarantini, S. et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glx177 (2017).
Ungvari, Z. et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 301, H363–H372 (2011).
Ungvari, Z. et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 66, 866–875 (2011).
Perridon, B. W., Leuvenink, H. G., Hillebrands, J. L., van Goor, H. & Bos, E. M. The role of hydrogen sulfide in aging and age-related pathologies. Aging 8, 2264–2289 (2016).
Yuan, S. et al. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol. 9, 157–166 (2016).
Hine, C. & Mitchell, J. R. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp. Gerontol. 68, 26–32 (2015).
Ungvari, Z. I. et al. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 293, H37–H47 (2007).
Sahoo, S., Meijles, D. N. & Pagano, P. J. NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 130, 317–335 (2016).
Csiszar, A., Wang, M., Lakatta, E. G. & Ungvari, Z. I. Inflammation and endothelial dysfunction during aging: role of NF-κB. J. Appl. Physiol. 105, 1333–1341 (2008).
Lee, I. M. & Paffenbarger, R. S. Jr. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke 29, 2049–2054 (1998).
Taddei, S. et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101, 2896–2901 (2000).
DeSouza, C. A. et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357 (2000).
Spier, S. A. et al. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 292, H3119–H3127 (2007).
Trott, D. W., Gunduz, F., Laughlin, M. H. & Woodman, C. R. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J. Appl. Physiol. 106, 1925–1934 (2009).
Durrant, J. R. et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J. Physiol. 587, 3271–3285 (2009).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Ma, L. et al. Anti-peroxynitrite treatment ameliorated vasorelaxation of resistance arteries in aging rats: involvement with NO-sGC-cGKs pathway. PLoS ONE 9, e104788 (2014).
Xu, S. et al. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 290, H2220–H2227 (2006).
Burkle, A., Beneke, S. & Muiras, M. L. Poly(ADP-ribosyl)ation and aging. Exp. Gerontol. 39, 1599–1601 (2004).
Pacher, P. et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J. Pharmacol. Exp. Ther. 311, 485–491 (2004).
Pacher, P. et al. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br. J. Pharmacol. 135, 1347–1350 (2002).
Ha, H. C., Hester, L. D. & Snyder, S. H. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc. Natl Acad. Sci. USA 99, 3270–3275 (2002).
Carrillo, A. et al. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 32, 757–766 (2004).
Andreone, T. L., O’Connor, M., Denenberg, A., Hake, P. W. & Zingarelli, B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J. Immunol. 170, 2113–2120 (2003).
Zingarelli, B. et al. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol. Med. 9, 143–153 (2003).
Hassa, P. O. & Hottiger, M. O. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol. Chem. 380, 953–959 (1999).
Pacher, P. & Szabo, C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 173, 2–13 (2008).
Berger, N. A. et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br. J. Pharmacol. 175, 192–222 (2018).
Baur, J. A., Ungvari, Z., Minor, R. K., Le Couteur, D. G. & de Cabo, R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 11, 443–461 (2012).
Gano, L. B. et al. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 307, H1754–H1763 (2014).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Lynch, C. D. et al. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol. Aging 20, 191–200 (1999).
Csiszar, A. et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 307, H292–H306 (2014).
Csiszar, A. et al. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 68, 235–249 (2013).
Katare, R. G., Kakinuma, Y., Arikawa, M., Yamasaki, F. & Sato, T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J. Mol. Cell. Cardiol. 46, 405–412 (2009).
Pierce, G. L. et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52, 72–79 (2008).
Lesniewski, L. A., Zigler, M. C., Durrant, J. R., Donato, A. J. & Seals, D. R. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech. Ageing Dev. 133, 368–371 (2012).
Lin, A. L. et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow Metab. 33, 1412–1421 (2013).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Dai, D. F. et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014).
Lesniewski, L. A. et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16, 17–26 (2017).
Lin, A. L. et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J. Cereb. Blood Flow Metab. 37, 217–226 (2017).
Durik, M. et al. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation 126, 468–478 (2012).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Bhayadia, R., Schmidt, B. M., Melk, A. & Homme, M. Senescence-induced oxidative stress causes endothelial dysfunction. J. Gerontol. A Biol. Sci. Med. Sci. 71, 161–169 (2016).
Ungvari, Z. et al. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience 39, 33–42 (2017).
LaRocca, T. J. et al. Translational evidence that impaired autophagy contributes to arterial ageing. J. Physiol. 590, 3305–3316 (2012).
Masser, D. R. et al. Analysis of DNA modifications in aging research. Geroscience 40, 11–29 (2018).
Benayoun, B. A., Pollina, E. A. & Brunet, A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610 (2015).
Unnikrishnan, A. et al. Revisiting the genomic hypomethylation hypothesis of aging. Ann. NY Acad. Sci. https://doi.org/10.1111/nyas.13533 (2018).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Man, H. S., Yan, M. S., Lee, J. J. & Marsden, P. A. Epigenetic determinants of cardiovascular gene expression: vascular endothelium. Epigenomics 8, 959–979 (2016).
Galonska, C. et al. Genome-wide tracking of dCas9-methyltransferase footprints. Nat. Commun. 9, 597 (2018).
Toth, P. et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14, 1034–1044 (2015).
Sonntag, W. E. et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 5, 27 (2013).
Bailey-Downs, L. C. et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J. Gerontol. Biol. Med. Sci. 67, 313–329 (2012).
Csiszar, A. et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am. J. Physiol. Heart Circ. Physiol. 295, H1882–H1894 (2008).
Tarnawski, A. S., Pai, R., Tanigawa, T., Matysiak-Budnik, T. & Ahluwalia, A. PTEN silencing reverses aging-related impairment of angiogenesis in microvascular endothelial cells. Biochem. Biophys. Res. Commun. 394, 291–296 (2010).
Bach, M. H., Sadoun, E. & Reed, M. J. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech. Ageing Dev. 126, 467–473 (2005).
Sadoun, E. & Reed, M. J. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J. Histochem. Cytochem. 51, 1119–1130 (2003).
Ahluwalia, A. & Tarnawski, A. S. Activation of the metabolic sensor — AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J. Physiol. Pharmacol. 62, 583–587 (2011).
Lahteenvuo, J. & Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 110, 1252–1264 (2012).
Ingraham, J. P., Forbes, M. E., Riddle, D. R. & Sonntag, W. E. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J. Gerontol. A Biol. Sci. Med. Sci. 63, 12–20 (2008).
Anversa, P., Li, P., Sonnenblick, E. H. & Olivetti, G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am. J. Physiol. 267, H1062–H1073 (1994).
Murugesan, N., Demarest, T. G., Madri, J. A. & Pachter, J. S. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol. Aging 33, 1004.e1–1004.e16 (2011).
Benderro, G. F. & Lamanna, J. C. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 1389, 50–60 (2011).
Goligorsky, M. S. Microvascular rarefaction: the decline and fall of blood vessels. Organogenesis 6, 1–10 (2010).
Ungvari, Z. et al. Aging-induced dysregulation of Dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 68, 877–891 (2013).
Ziche, M. et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Invest. 99, 2625–2634 (1997).
Sladek, T., Gerova, M., Znojil, V. & Devat, L. Morphometric characteristics of cardiac hypertrophy induced by long-term inhibition of NO synthase. Physiol. Res. 45, 335–338 (1996).
Kubis, N., Richer, C., Domergue, V., Giudicelli, J. F. & Levy, B. I. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt−/−). J. Hypertens. 20, 1581–1587 (2002).
Oomen, C. A. et al. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front. Aging Neurosci. 1, 4 (2009).
Sawada, N. & Arany, Z. Metabolic regulation of angiogenesis in diabetes and aging. Physiology 32, 290–307 (2017).
Maizel, J. et al. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 307, H1691–H1704 (2014).
Ahluwalia, A. & Tarnawski, A. S. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J. Physiol. Pharmacol. 62, 583–587 (2011).
de Lucia, C. et al. microRNA in cardiovascular aging and age-related cardiovascular diseases. Front. Med. 4, 74 (2017).
Bonauer, A. et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324, 1710–1713 (2009).
Doebele, C. et al. Members of the microRNA-17-92 cluster exhibit a cell intrinsic anti-angiogenic function in endothelial cells. Blood 115, 4944–4950 (2010).
Kuehbacher, A., Urbich, C., Zeiher, A. M. & Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 101, 59–68 (2007).
Suarez, Y., Fernandez-Hernando, C., Pober, J. S. & Sessa, W. C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 100, 1164–1173 (2007).
Suarez, Y. et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl Acad. Sci. USA 105, 14082–14087 (2008).
Yang, W. J. et al. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 280, 9330–9335 (2005).
Yan, Y. et al. Dicer expression exhibits a tissue-specific diurnal pattern that is lost during aging and in diabetes. PLoS ONE 8, e80029 (2013).
Che, P. et al. miR-125a-5p impairs endothelial cell angiogenesis in aging mice via RTEF-1 downregulation. Aging Cell 13, 926–934 (2014).
Wei, Y. et al. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc. Natl Acad. Sci. USA 110, E3910–E3918 (2013).
Valcarcel-Ares, M. N. et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 67, 821–829 (2012).
Ungvari, Z. et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1443–1457 (2013).
Warrington, J. P. et al. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J. Vasc. Res. 50, 445–457 (2013).
Roos, C. M. et al. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. Am. J. Physiol. Heart Circ. Physiol. 305, H1428–H1439 (2013).
Franco, S., Segura, I., Riese, H. H. & Blasco, M. A. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 62, 552–559 (2002).
Murasawa, S. et al. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation 106, 1133–1139 (2002).
Csiszar, A., Ungvari, Z., Koller, A., Edwards, J. G. & Kaley, G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol. Genom. 17, 21–30 (2004).
Pearson, K. J. et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 (2008).
Khan, A. S., Lynch, C. D., Sane, D. C., Willingham, M. C. & Sonntag, W. E. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 56, B364–B371 (2001).
Tarantini, S. et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age 38, 273–289 (2016).
Sonntag, W. E., Lynch, C. D., Cooney, P. T. & Hutchins, P. M. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 138, 3515–3520 (1997).
Scheubel, R. J. et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 42, 2073–2080 (2003).
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014).
Tucsek, Z. et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1339–1352 (2014).
van Almen, G. C. et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 10, 769–779 (2011).
Wagatsuma, A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp. Gerontol. 41, 49–54 (2006).
Ryan, N. A. et al. Lower skeletal muscle capillarization and VEGF expression in aged versus young men. J. Appl. Physiol. 100, 178–185 (2006).
Iemitsu, M., Maeda, S., Jesmin, S., Otsuki, T. & Miyauchi, T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am. J. Physiol. Heart Circ. Physiol. 291, H1290–H1298 (2006).
Mieno, S. et al. Aging is associated with an impaired coronary microvascular response to vascular endothelial growth factor in patients. J. Thorac. Cardiovasc. Surg. 132, 1348–1355 (2006).
Sarzani, R., Arnaldi, G., Takasaki, I., Brecher, P. & Chobanian, A. V. Effects of hypertension and aging on platelet-derived growth factor and platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension 18 (Suppl.), III93–III99 (1991).
Banki, E. et al. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 70, 665–674 (2015).
Bearzi, C. et al. Identification of a coronary vascular progenitor cell in the human heart. Proc. Natl Acad. Sci. USA 106, 15885–15890 (2009).
Chang, E. I. et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 116, 2818–2829 (2007).
Heiss, C. et al. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 45, 1441–1448 (2005).
Thijssen, D. H. et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell 5, 495–503 (2006).
Keymel, S. et al. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Bas. Res. Cardiol. 103, 582–586 (2008).
He, T., Joyner, M. J. & Katusic, Z. S. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc. Res. 78, 447–452 (2009).
Kushner, E. J. et al. Aging is associated with a proapoptotic endothelial progenitor cell phenotype. J. Vasc. Res. 48, 408–414 (2011).
Turgeon, J. et al. Protection against vascular aging in Nox2-deficient mice: impact on endothelial progenitor cells and reparative neovascularization. Atherosclerosis 223, 122–129 (2012).
Thum, T. et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ. Res. 100, 434–443 (2007).
Hoetzer, G. L. et al. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J. Appl. Physiol. 102, 847–852 (2007).
Zhu, G. et al. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann. Vasc. Surg. 23, 519–534 (2009).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Kelly-Goss, M. R., Sweat, R. S., Stapor, P. C., Peirce, S. M. & Murfee, W. L. Targeting pericytes for angiogenic therapies. Microcirculation 21, 345–357 (2014).
Stapor, P. C., Sweat, R. S., Dashti, D. C., Betancourt, A. M. & Murfee, W. L. Pericyte dynamics during angiogenesis: new insights from new identities. J. Vasc. Res. 51, 163–174 (2014).
Toth, P. et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. 33, 1732–1742 (2013).
Stefanska, A. et al. Interstitial pericytes decrease in aged mouse kidneys. Aging 7, 370–382 (2015).
Hughes, S. et al. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol. Aging 27, 1838–1847 (2006).
Yang, J. et al. Synchronized age-related gene expression changes across multiple tissues in human and the link to complex diseases. Sci. Rep. 5, 15145 (2015).
Acknowledgements
The authors are supported by grants from the AHA (S.T.), the US National Institute on Aging (R01-AG055395, R01-AG047879, R01-AG038747, P30-AG050911, and R01AG049821), the US National Institute of Neurological Disorders and Stroke (R01-NS056218 and R01-NS100782), the US National Heart, Lung, and Blood Institute (R01-HL111178 and R01-HL134778), the Oklahoma Center for the Advancement of Science and Technology, and the Presbyterian Health Foundation. The authors acknowledge support from the National Institute on Aging-funded Geroscience Training Program in Oklahoma, USA (T32AG052363), and the EU-funded EFOP-3.6.1-16-2016-0008 programme.
Reviewer information
Nature Reviews Cardiology thanks J. Padilla and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, discussed its content, wrote the manuscript, and reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ungvari, Z., Tarantini, S., Kiss, T. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15, 555–565 (2018). https://doi.org/10.1038/s41569-018-0030-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0030-z
This article is cited by
-
Effect of aging on the human myometrium at single-cell resolution
Nature Communications (2024)
-
Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging
Nature Aging (2024)
-
A high-resolution view of the heterogeneous aging endothelium
Angiogenesis (2024)
-
Endothelial dysfunction and cardiovascular risk in post-COVID-19 patients after 6- and 12-months SARS-CoV-2 infection
Infection (2024)
-
Impaired angiogenesis in ageing: the central role of the extracellular matrix
Journal of Translational Medicine (2023)