Abstract
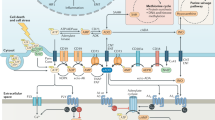
Modulation of the biochemical composition of the tumour microenvironment is a new frontier of cancer therapy. Several immunosuppressive mechanisms operate in the milieu of most tumours, a condition that makes antitumour immunity ineffective. One of the most potent immunosuppressive factors is adenosine, which is generated in the tumour microenvironment owing to degradation of extracellular ATP. Accruing evidence over the past few years shows that ATP is one of the major biochemical constituents of the tumour microenvironment, where it acts at P2 purinergic receptors expressed on both tumour and host cells. Stimulation of P2 receptors has different effects depending on the extracellular ATP concentration, the P2 receptor subtype engaged and the target cell type. Among P2 receptors, the P2X purinergic receptor 7 (P2X7R) subtype appears to be a main player in host–tumour cell interactions. Preclinical studies in several tumour models have shown that P2X7R targeting is potentially a very effective anticancer treatment, and many pharmaceutical companies have now developed potent and selective small molecule inhibitors of P2X7R. In this Review, we report on the multiple mechanisms by which extracellular ATP shapes the tumour microenvironment and how its stimulation of host and tumour cell P2 receptors contributes to determining tumour fate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weber, G. F. Time and circumstances: cancer cell metabolism at various stages of disease progression. Front. Oncol. 6, 257 (2016).
Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007).
Burnstock, G. & Di Virgilio, F. Purinergic signalling and cancer. Purinergic Signal. 9, 491–540 (2013).
Forrester, T. A case of serendipity*. Purinergic Signal. 4, 93–100 (2008).
Pellegatti, P. et al. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLOS ONE 3, e2599 (2008). This study demonstrates in vivo application of the genetically encoded probe pmeLUC and reports that extracellular ATP is dramatically increased in the TME.
Morciano, G. et al. Use of luciferase probes to measure ATP in living cells and animals. Nat. Protoc. 12, 1542–1562 (2017).
Ohta, A. et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA 103, 13132–13137 (2006).
Vijayan, D., Young, A., Teng, M. W. L. & Smyth, M. J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017).
Burnstock, G. Purinergic nerves. Pharmacol. Rev. 24, 509–581 (1972). The study marks the beginning of modern purinergic signalling research.
Cockcroft, S. & Gomperts, B. D. The ATP4- receptor of rat mast cells. Biochem. J. 188, 789–798 (1980). This study provides rigorous characterization of ATP-induced changes in plasma membrane permeability and postulates the expression of specific plasma membrane receptors for extracellular ATP.
Rapaport, E., Fishman, R. F. & Gercel, C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5′-triphosphate. Cancer Res. 43, 4402–4406 (1983).
Nolde, S. & Hilz, H. The cytostatic action of extracellular NAD in tumour-bearing mice. Br. J. Cancer 26, 299–303 (1972).
Rozengurt, E. & Heppel, L. A. A specific effect of external ATP on the permeability of transformed 3T3 cells. Biochem. Biophys. Res. Commun. 67, 1581–1588 (1975).
Kepp, O., Loos, F., Liu, P. & Kroemer, G. Extracellular nucleosides and nucleotides as immunomodulators. Immunol. Rev. 280, 83–92 (2017).
Di Virgilio, F. & Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 36, 293–303 (2017).
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L. & Falzoni, S. The P2X7 receptor in infection and inflammation. Immunity 47, 15–31 (2017). This is a thorough review that reports the molecular structure and pathophysiological function of P2X7R.
Lustig, K. D., Shiau, A. K., Brake, A. J. & Julius, D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl Acad. Sci. USA 90, 5113–5117 (1993).
Webb, T. E. et al. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 324, 219–225 (1993).
Brake, A. J., Wagenbach, M. J. & Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523 (1994).
Jacobson, K. A. & Muller, C. E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104, 31–49 (2016).
North, R. A. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 (2002).
Baroja-Mazo, A., Barbera-Cremades, M. & Pelegrin, P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta 1828, 79–93 (2013).
Karasawa, A., Michalski, K., Mikhelzon, P. & Kawate, T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. eLife 6, e31186 (2017). This is a fundamental study that highlights the role of plasma membrane cholesterol in the regulation of P2X7R function and sheds light on the elusive mechanism of P2X7R-associated increases in plasma membrane permeability and the role of the carboxy-terminal tail of the P2X7 subunit.
Pippel, A. et al. Localization of the gate and selectivity filter of the full-length P2X7 receptor. Proc. Natl Acad. Sci. USA 114, E2156–E2165 (2017).
Di Virgilio, F., Schmalzing, G. & Markwardt, F. The elusive P2X7 macropore. Trends Cell Biol. 28, 392–404 (2018). This is an updated review of the most recent studies investigating the molecular mechanism underlying the formation of the P2X7R macropore.
Khakh, B. S. & Lester, H. A. Dynamic selectivity filters in ion channels. Neuron 23, 653–658 (1999).
Venereau, E., Ceriotti, C. & Bianchi, M. E. DAMPs from cell death to new life. Front. Immunol. 6, 422 (2015).
McAllister, S. S. & Weinberg, R. A. Tumor-host interactions: a far-reaching relationship. J. Clin. Oncol. 28, 4022–4028 (2010).
Munn, D. H. & Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39, 1–6 (2016).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Di Virgilio, F., Boeynaems, J. M. & Robson, S. C. Extracellular nucleotides as negative modulators of immunity. Curr. Opin. Pharmacol. 9, 507–513 (2009).
Klement, G. L. Eco-evolution of cancer resistance. Sci. Transl Med. 8, 327fs5 (2016).
Gareau, A. J. et al. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin. Exp. Metastasis 35, 25–35 (2018).
Arulkumaran, N., Unwin, R. J. & Tam, F. W. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin. Investig. Drugs 20, 897–915 (2011).
Park, J. H. & Kim, Y. C. P2X7 receptor antagonists: a patent review. Expert Opin. Ther. Pat. 27, 257–267 (2017).
Lecciso, M. et al. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front. Immunol. 8, 1918 (2017).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011). This study shows that autophagy drives ATP release into the TME and that an increased ATP level in the TME is needed for an efficient chemotherapeutic response.
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Loo, J. M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
Chen, Y. et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 (2006).
Conley, J. M., Radhakrishnan, S., Valentino, S. A. & Tantama, M. Imaging extracellular ATP with a genetically-encoded, ratiometric fluorescent sensor. PLOS ONE 12, e0187481 (2017).
Beigi, R., Kobatake, E., Aizawa, M. & Dubyak, G. R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 276, C267–C278 (1999).
Hatfield, S. M. et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 7, 277ra30 (2015).
Forrester, T. & Williams, C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J. Physiol. 268, 371–390 (1977).
Milo, R., Jorgensen, P., Moran, U., Weber, G. & Springer, M. BioNumbers — the database of key numbers in molecular and cell biology. Nucleic Acids Res. 38, D750–D753 (2010).
Lazarowski, E. R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8, 359–373 (2012).
Evans, R. J., Derkach, V. & Surprenant, A. ATP mediates fast synaptic transmission in mammalian neurons. Nature 357, 503–505 (1992).
Pettersson, H. et al. SLC10A4 regulates IgE-mediated mast cell degranulation in vitro and mast cell-mediated reactions in vivo. Sci. Rep. 7, 1085 (2017).
Sawada, K. et al. Identification of a vesicular nucleotide transporter. Proc. Natl Acad. Sci. USA 105, 5683–5686 (2008).
Tokunaga, A., Tsukimoto, M., Harada, H., Moriyama, Y. & Kojima, S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J. Biol. Chem. 285, 17406–17416 (2010).
Oviedo-Orta, E. & Howard, E. W. Gap junctions and connexin-mediated communication in the immune system. Biochim. Biophys. Acta 662, 102–112 (2004).
Adamson, S. E. & Leitinger, N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 588, 1416–1422 (2014).
Locovei, S., Wang, J. & Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244 (2006).
Qiu, F. & Dahl, G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell Physiol. 296, C250–C255 (20=9).
Yang, D., He, Y., Munoz-Planillo, R., Liu, Q. & Nunez, G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932 (2015).
Boyd-Tressler, A., Penuela, S., Laird, D. W. & Dubyak, G. R. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J. Biol. Chem. 289, 27246–27263 (2014).
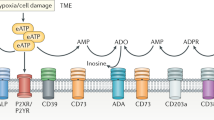
Zimmermann, H., Zebisch, M. & Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502 (2012).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Eltzschig, H. K. et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113, 224–232 (2009).
Li, J. et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 6, e1320011 (2017).
Dwyer, K. M. et al. CD39 and control of cellular immune responses. Purinergic Signal. 3, 171–180 (2007).
Chalmin, F. et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362–373 (2012).
Montalban, D. B. et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages — a self-amplifying, CD39- and CD73- dependent mechanism for tumor immune escape. J. Immunother. Cancer 4, 49 (2016).
Ryzhov, S. V. et al. Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors. J. Immunol. 193, 3155–3164 (2014).
Molinier-Frenkel, V. & Castellano, F. Immunosuppressive enzymes in the tumor microenvironment. FEBS Lett. 591, 3135–3157 (2017).
Jackson, S. W. et al. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am. J. Pathol. 171, 1395–1404 (2007).
Stagg, J. et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 71, 2892–2900 (2011).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
Yegutkin, G. G., Samburski, S. S., Mortensen, S. P., Jalkanen, S. & Gonzalez-Alonso, J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J. Physiol. 579, 553–564 (2007).
Yegutkin, G. G., Samburski, S. S. & Jalkanen, S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 17, 1328–1330 (2003).
Banz, Y. et al. CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br. J. Haematol. 142, 627–637 (2008).
Qian, Y. et al. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 351, 242–251 (2014).
Wang, X. et al. Extracellular ATP, as an energy and phosphorylating molecule, induces different types of drug resistances in cancer cells through ATP internalization and intracellular ATP level increase. Oncotarget 8, 87860–87877 (2017).
Allard, B., Beavis, P. A., Darcy, P. K. & Stagg, J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 29, 7–16 (2016).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Allard, B., Longhi, M. S., Robson, S. C. & Stagg, J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 (2017).
Junger, W. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201-212 (2011). This study provides an in depth and authoritative analysis of the role of nucleotides as chemoattractants in inflammation and infection.
Trabanelli, S. et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J. Immunol. 189, 1303–1310 (2012).
Ferrari, D. et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp. Hematol. 39, 360–374 (2011).
Idzko, M., Ferrari, D. & Eltzschig, H. K. Nucleotide signalling during inflammation. Nature 509, 310–317 (2014). This authoritative review describes the role of extracellular nucleotide signalling in inflammation and immunity, with reference to relevant disease states.
Saez, P. J. et al. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal. 10, eaah7107 (2017).
Chiu, D. K. et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 8, 517 (2017).
Bianchi, G. et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis. 5, e1135 (2014).
Goldszmid, R. S., Dzutsev, A. & Trinchieri, G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 15, 295–305 (2014).
la Sala, A. et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 166, 1611–1617 (2001).
la Sala, A. et al. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1T lymphocytes. Blood 99, 1715–1722 (2002).
Ferrari, D. et al. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 14, 2466–2476 (2000).
Idzko, M. et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood 100, 925–932 (2002).
Mutini, C. et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J. Immunol. 163, 1958–1965 (1999).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009). This study demonstrates the role played by the NLRP3 inflammasome and P2X7R in promoting anticancer immunity in the TME.
Aswad, F., Kawamura, H. & Dennert, G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J. Immunol. 175, 3075–3083 (2005).
Dang, E. V. et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Amoroso, F. et al. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene 34, 5240–5251 (2015).
Gu, B. J. & Wiley, J. S. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 107, 4946–4953 (2006).
Lopez-Castejon, G. et al. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J. Immunol. 185, 2611–2619 (2010).
Amoroso, F., Falzoni, S., Adinolfi, E., Ferrari, D. & Di Virgilio, F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 3, e370 (2012).
Vaupel, P. & Multhoff, G. Accomplices of the hypoxic tumor microenvironment compromising antitumor immunity: adenosine, lactate, acidosis, vascular endothelial growth factor, potassium ions, and phosphatidylserine. Front. Immunol. 8, 1887 (2017).
Pirotton, S., Communi, D., Motte, S., Janssens, R. & Boeynaems, J. M. Endothelial P2-purinoceptors: subtypes and signal transduction. J. Auton. Pharmacol. 16, 353–356 (1996).
Rumjahn, S. M., Yokdang, N., Baldwin, K. A., Thai, J. & Buxton, I. L. Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. Br. J. Cancer 100, 1465–1470 (2009).
Adinolfi, E. et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 72, 2957–2969 (2012). This study provides in vivo demonstration of the tumour growth-promoting activity of P2X7R and of the efficacy of P2X7R-targeted drugs as an antitumour therapy.
Hill, L. M., Gavala, M. L., Lenertz, L. Y. & Bertics, P. J. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J. Immunol. 185, 3028–3034 (2010).
Pfeiffer, Z. A. et al. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic. Biol. Med. 42, 1506–1516 (2007).
Furlan-Freguia, C., Marchese, P., Gruber, A., Ruggeri, Z. M. & Ruf, W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J. Clin. Invest. 121, 2932–2944 (2011).
Bubici, C. & Papa, S. JNK signalling in cancer: in need of new, smarter therapeutic targets. Br. J. Pharmacol. 171, 24–37 (2014).
Yu, J. L. et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105, 1734–1741 (2005).
Adinolfi, E. et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 99, 706–708 (2002).
McLarnon, J. G. Roles of purinergic P2X7 receptor in glioma and microglia in brain tumors. Cancer Lett. 402, 93–99 (2017).
Solini, A. et al. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology 149, 389–396 (2008).
Schmid, S. et al. Altered purinergic signaling in the tumor associated immunologic microenvironment in metastasized non-small-cell lung cancer. Lung Cancer 90, 516–521 (2015).
Zhang, X. J. et al. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk. Res. 28, 1313–1322 (2004).
Jiang, X. et al. Silencing P2X7 receptor downregulates the expression of TCP-1 involved in lymphoma lymphatic metastasis. Oncotarget 6, 42105–42117 (2015).
Salvestrini, V. et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 8, 5895–5908 (2017).
Baricordi, O. R. et al. Increased proliferation rate of lymphoid cells transfected with the P2X7 ATP receptor. J. Biol. Chem. 274, 33206–33208 (1999). This study reports the paradoxical finding that expression of P2X7R, far from increasing spontaneous cell death, increased cell survival and proliferation.
Adinolfi, E. et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol. Biol. Cell 16, 3260–3272 (2005).
Vazquez-Cuevas, F. G. et al. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J. Cell. Biochem. 115, 1955–1966 (2014).
Adinolfi, E. et al. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1 and protects from apoptosis. J. Biol. Chem. 284, 10120–10128 (2009).
Adinolfi, E. et al. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 75, 635–644 (2015).
Amoroso, F. et al. P2X7 targeting inhibits growth of human mesothelioma. Oncotarget 7, 49664–49676 (2016).
Adinolfi, E. et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 24, 3393–3404 (2010).
Giuliani, A. L. et al. Trophic activity of human P2X7 receptor isoforms A and B in osteosarcoma. PLOS ONE 9, e107224 (2014).
Sluyter, R. The P2X7 receptor. Adv. Exp. Med. Biol. 1051, 17–53 (2017). This study provides a fundamental review of the genetics, molecular structure, pharmacology, physiology and pathophysiology of human and mouse P2X7R, with valuable information about this receptor in other species.
Burnstock, G. & Knight, G. E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 14, 1–18 (2018).
De Marchi, E., Orioli, E., Dal Ben, D. & Adinolfi, E. P2X7 receptor as a therapeutic target. Adv. Protein Chem. Struct. Biol. 104, 39–79 (2016).
Raffaghello, L., Chiozzi, P., Falzoni, S., Di Virgilio, F. & Pistoia, V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 66, 907–914 (2006).
Beloribi-Djefaflia, S., Vasseur, S. & Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189 (2016).
Li, Y. C., Park, M. J., Ye, S. K., Kim, C. W. & Kim, Y. N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 168, 1107–1118 (2006).
Robinson, L. E., Shridar, M., Smith, P. & Murrell-Lagnado, R. D. Plasma membrane cholesterol as a regulator of human and rodent P2X7 receptor activation and sensitization. J. Biol. Chem. 289, 31983–31994 (2014). This study reports a thorough characterization of the effect of plasma membrane cholesterol on the function of P2XRs and paves the way to understanding how these receptors can be modulated by membrane lipids.
Wan, H. X., Hu, J. H., Xie, R., Yang, S. M. & Dong, H. Important roles of P2Y receptors in the inflammation and cancer of digestive system. Oncotarget 7, 28736–28747 (2016).
Tarasov, A. I., Griffiths, E. J. & Rutter, G. A. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52, 28–35 (2012).
Bao, Y. et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J. Biol. Chem. 289, 26794–26803 (2014).
Ledderose, C. et al. Cutting off the power: inhibition of leukemia cell growth by pausing basal ATP release and P2X receptor signaling? Purinergic Signal. 12, 439–451 (2016).
Draoui, N., de Zeeuw, P. & Carmeliet, P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 7, 170219 (2017).
Mowers, E. E., Sharifi, M. N. & Macleod, K. F. Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 285,1751–1766 (2018).
Orioli, E., De Marchi, E., Giuliani, A. L. & Adinolfi, E. P2X7 receptor orchestrates multiple signalling pathways triggering inflammation, autophagy and metabolic/trophic responses. Curr. Med. Chem. 24, 2261–2275 (2017).
Bian, S. et al. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLOS ONE 8, e60184 (2013).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). This report provides a thorough and authoritative description of the process of ICD and its implications for cancer therapy.
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Wang, Y. et al. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 9, 1624–1625 (2013).
Pietrocola, F., Bravo-San Pedro, J. M., Galluzzi, L. & Kroemer, G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy 13, 263–2170 (2017).
Galluzzi, L. et al. Classification of current anticancer immunotherapies. Oncotarget 5, 12472–12508 (2014).
Garg, A. D., Martin, S., Golab, J. & Agostinis, P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. 21, 26–38 (2014).
Michaud, M. et al. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 1, 393–395 (2012).
Ohshima, Y. et al. γ-Irradiation induces P2X7 receptor-dependent ATP release from B16 melanoma cells. Biochim. Biophys. Acta 1800, 40–46 (2010).
Clark, A. G. & Vignjevic, D. M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 36, 13–22 (2015).
Friedl, P. & Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011).
McCawley, L. J. & Matrisian, L. M. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol. Med. Today 6, 149–156 (2000).
Di Virgilio, F., Falzoni, S., Giuliani, A. L. & Adinolfi, E. P2 receptors in cancer progression and metastatic spreading. Curr. Opin. Pharmacol. 29, 17–25 (2016).
Jelassi, B. et al. P2X7 receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 30, 2108–2122 (2011).
Qiu, Y. et al. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLOS ONE 9, e114371 (2014).
Xia, J., Yu, X., Tang, L., Li, G. & He, T. P2X7 receptor stimulates breast cancer cell invasion and migration via the AKT pathway. Oncol. Rep. 34, 103–110 (2015).
Jelassi, B. et al. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 34, 1487–1496 (2013).
Takai, E. et al. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J. Cell Sci. 125, 5051–5060 (2012).
Takai, E., Tsukimoto, M., Harada, H. & Kojima, S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 10, 487–497 (2014).
Giannuzzo, A., Pedersen, S. F. & Novak, I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol. Cancer 14, 203 (2015).
Park, J. H. et al. Potent suppressive effects of 1-piperidinylimidazole based novel P2X7 receptor antagonists on cancer cell migration and invasion. J. Med. Chem. 59, 7410–7430 (2016).
Gu, L. Q. et al. Association of XIAP and P2X7 receptor expression with lymph node metastasis in papillary thyroid carcinoma. Endocrine 38, 276–282 (2010).
Li, W. H. et al. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br. J. Cancer 109, 1666–1675 (2013).
Li, W. H., Qiu, Y., Zhang, H. Q., Tian, X. X. & Fang, W. G. P2Y2 receptor and EGFR cooperate to promote prostate cancer cell invasion via ERK1/2 pathway. PLOS ONE 10, e0133165 (2015).
Lamarca, A. et al. Uridine 5′-triphosphate promotes in vitro Schwannoma cell migration through matrix metalloproteinase-2 activation. PLOS ONE 9, e98998 (2014).
Khalid, M. et al. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells. Oncotarget 8, 37278–37290 (2017).
MacKenzie, A. et al. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15, 825–835 (2001). This paper shows that P2X7R activation is a potent stimulus for the release of plasma membrane-derived microvesicles loaded with IL-1β, thus suggesting a pathway for the release of this cytokine during inflammation.
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Dubyak, G. R. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell. Microbiol. 14, 1697–1706 (2012).
Baroni, M. et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 21, 1926–1933 (2007).
Wendler, F. et al. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene 36, 877–884 (2017).
Lobb, R. J., Lima, L. G. & Moller, A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 67, 3–10 (2017).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Chadet, S. et al. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis 35, 1238–1247 (2014).
Eddy, R. J., Weidmann, M. D., Sharma, V. P. & Condeelis, J. S. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 27, 595–607 (2017).
Verhoef, P. A., Estacion, M., Schilling, W. & Dubyak, G. R. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J. Immunol. 170, 5728–5738 (2003).
Morelli, A. et al. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol. Biol. Cell 14, 2655–2664 (2003).
Mackenzie, A. B., Young, M. T., Adinolfi, E. & Surprenant, A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J. Biol. Chem. 280, 33968–33976 (2005).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Cho, M. S. et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood 130, 1235–1242 (2017).
Meyers, K. M., Holmsen, H. & Seachord, C. L. Comparative study of platelet dense granule constituents. Am. J. Physiol. 243, R454–R461 (1982).
Schumacher, D., Strilic, B., Sivaraj, K. K., Wettschureck, N. & Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24, 130–137 (2013). This study describes the mechanism by which ATP release from platelets bound to circulating cancer cells activates P2Y2R expressed on the plasma membrane of endothelial cells, thus promoting the opening of inter-endothelial junctions and cancer cell extravasation.
Burnstock, G. Purinergic signaling in the cardiovascular system. Circ. Res. 120, 207–228 (2017).
Stanger, B. Z. & Kahn, M. L. Platelets and tumor cells: a new form of border control. Cancer Cell 24, 9–11 (2013).
Gasic, G. J., Gasic, T. B. & Stewart, C. C. Antimetastatic effects associated with platelet reduction. Proc. Natl Acad. Sci. USA 61, 46–52 (1968).
Gay, L. J. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 (2011). This study provides an excellent appraisal of the role of platelets in cancer metastasis.
Xie, R. et al. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J. Biol. Chem. 289, 19137–19149 (2014).
Shabbir, M., Thompson, C., Jarmulowiczc, M., Mikhailidis, D. & Burnstock, G. Effect of extracellular ATP on the growth of hormone-refractory prostate cancer in vivo. BJU Int. 102, 108–112 (2008).
Furlow, P. W. et al. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat. Cell Biol. 17, 943–952 (2015).
Burma, N. E. et al. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat. Med. 23, 355–360 (2017).
Marcus, A. J. et al. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 305, 9–16 (2003).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00471120 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02293811 (2014).
Slater, M. et al. Differentiation between cancerous and normal hyperplastic lobules in breast lesions. Breast Cancer Res. Treat. 83, 1–10 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02587819 (2016).
Sainz, B. Jr et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut 64, 1921–1935 (2015).
Srivastava, P. et al. ATP-decorated mesoporous silica for biomineralization of calcium carbonate and P2 purinergic receptor-mediated antitumor activity against aggressive lymphoma. ACS Appl. Mater. Interfaces 10, 6917–6929 (2018).
Danquah, W. et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci. Transl. Med 8, 366ra162 (2016). This study reports the first application of anti-P2X7R nanobodies in an in vivo model of inflammation.
Bannas, P., Hambach, J. & Koch-Nolte, F. Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Front. Immunol. 8, 1603 (2017).
de Andrade, M. P. et al. Hyperthermia and associated changes in membrane fluidity potentiate P2X7 activation to promote tumor cell death. Oncotarget 8, 67254–67268 (2017).
Abdulqawi, R. et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205 (2015).
Tsuda, M. et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003).
Nippon Chemiphar Pipeline. Nippon Chemiphar http://www.chemiphar.co.jp/english/company/pipeline.html (2018).
Hechler, B. & Gachet, C. Purinergic receptors in thrombosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 35, 2307–2315 (2015).
Angiolillo, D. J. et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 136, 1955–1975 (2017).
Wang, Y. et al. Platelet P2Y12 is involved in murine pulmonary metastasis. PLOS ONE 8, e80780 (2013).
Kamiyama, M. et al. ASK1 facilitates tumor metastasis through phosphorylation of an ADP receptor P2Y12 in platelets. Cell Death Differ. 24, 2066–2076 (2017).
Elaskalani, O., Falasca, M., Moran, N., Berndt, M. C. & Metharom, P. The role of platelet-derived ADP and ATP in promoting pancreatic cancer cell survival and gemcitabine resistance. Cancers 9, 142 (2017).
Gebremeskel, S., LeVatte, T., Liwski, R. S., Johnston, B. & Bezuhly, M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int. J. Cancer 136, 234–240 (2015).
Gresele, P., Momi, S., Malvestiti, M. & Sebastiano, M. Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev. 36, 331–355 (2017).
Serebruany, V. L., Cherepanov, V., Cabrera-Fuentes, H. A. & Kim, M. H. Solid cancers after antiplatelet therapy: confirmations, controversies, and challenges. Thromb. Haemost. 114, 1104–1112 (2015).
Serebruany, V. L. et al. Mortality and cancer after 12 versus 30 months dual antiplatelet therapy. The Korean Outcomes Registry Evaluating Antithrombotics (KOREA). Thromb. Haemost. 117, 934–939 (2017).
Leader, A. et al. The effect of combined aspirin and clopidogrel treatment on cancer incidence. Am. J. Med. 130, 826–832 (2017).
Hicks, B. M., Murray, L. J., Hughes, C. & Cardwell, C. R. Clopidogrel use and cancer-specific mortality: a population-based cohort study of colorectal, breast and prostate cancer patients. Pharmacoepidemiol. Drug Saf. 24, 830–840 (2015).
Kotronias, R. A. et al. Cancer event rate and mortality with thienopyridines: a systematic review and meta-analysis. Drug Saf. 40, 229–240 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02404363 (2017).
Hansen, R. R. et al. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur. J. Pharmacol. 688, 27–34 (2012).
Falk, S. et al. P2X7 receptor-mediated analgesia in cancer-induced bone pain. Neuroscience 291, 93–105 (2015).
Pellegatti, P., Falzoni, S., Pinton, P., Rizzuto, R. & Di Virgilio, F. A. Novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659–3665 (2005).
Suadicani, S. O., Brosnan, C. F. & Scemes, E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385 (2006).
Sorrentino, C., Miele, L., Porta, A., Pinto, A. & Morello, S. Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression. Oncotarget 7, 64274–64288 (2016).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Del Pozo, M. Y. et al. Mesenchymal cancer cell-stroma crosstalk promotes niche activation, epithelial reversion, and metastatic colonization. Cell Rep. 13, 2456–2469 (2015).
Coull, J. A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Gilbert, S. M. et al. A phase 1 clinical trial demonstrates nfP2X7 targeted antibodies provide a novel, safe and tolerable topical therapy for BCC. Br. J. Dermatol. 177, 117–124 (2017).
Hattori, F. et al. Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur. J. Pharmacol. 695, 20–26 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03280888 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00263211 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03245489 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00940784 (2014).
Mezouar, S., Darbousset, R., Dignat-George, F., Panicot-Dubois, L. & Dubois, C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int. J. Cancer 136, 462–475 (2015).
Pandey, A. et al. Anti-platelet agents augment cisplatin nanoparticle cytotoxicity by enhancing tumor vasculature permeability and drug delivery. Nanotechnology 25, 445101 (2014).
Food and Drug Administration. Briefing document, February 2009 meeting of FDA Cardiovascular and Renal Drugs Advisory Committee on prasugrel. FDA https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022307s000_sumr.pdf (2009).
Acknowledgements
F.D.V. is supported by grants from the Italian Association for Cancer Research (AIRC; IG 13025 and IG 18581) and the Ministry of Health of Italy (RF-2011-02348435). E.A. is supported by an AIRC Individual Grant (IG 16812). Both F.D.V. and E.A. are supported by institutional funds from the University of Ferrara. F.D.V. also acknowledges networking support from COST (European Cooperation in Science and Technology) Action BM-1406.
Reviewer information
Nature Reviews Cancer thanks S. Robson, J. Stagg and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
F.D.V. drafted sections and revised and wrote the final version of the article. E.A., S.F., A.C.S. and E.D.M. researched data and contributed some sections. All the authors contributed to reviewing and editing before submission.
Corresponding author
Ethics declarations
Competing interests
F.D.V. is a member of the Scientific Advisory Board of Biosceptre, a UK-based biotech company involved in the development of P2X purinergic receptor 7 (P2X7R)-targeted therapeutics. E.A., S.F., A.C.S. and E.D.M. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Metabotropic receptors
-
Plasma membrane receptors coupled to the generation of an intracellular second messenger.
- Ionotropic receptor
-
A plasma membrane receptor that, upon binding of an extracellular ligand, opens to enable transmembrane fluxes of small ions such as Na+, K+, Ca2+ and Cl−.
- Ectonucleotidases
-
Enzymes expressed on the surface of virtually all cell types, where they catalyse the removal of one or two phosphate residues from extracellular nucleotides to eventually generate nucleosides. Ectonucleotidases participate in multiple physiological and pathophysiological responses ranging from neurotransmission to hormone secretion and from inflammation to blood coagulation.
- Förster resonance energy transfer
-
(FRET). A physical property based on the measurement of light emission from a fluorescent molecule (acceptor) activated by an excited nearby molecule (donor) via a process of energy transfer (resonance) that does not involve emission of light.
- Maxi-anion channels
-
Ubiquitous large-conductance, anion-selective channels that are permeable to ATP and are activated by hypoxia, ischaemia and osmotic stress.
- Connexins
-
Proteins found in vertebrates that assemble to form gap junctions but may also function as hemi-channels in isolated cells.
- Pannexin 1
-
A member of the pannexin family of large transmembrane channels that are permeable to ions and small solutes (for example, ATP).
- Regulatory T cells
-
(Treg cells). A subpopulation of CD4+ T lymphocytes identified by the forkhead box protein P3 (FOXP3) transcription factor, which inhibit T cell functions via several mechanisms to down-modulate inflammation and prevent autoimmune responses. Treg cells are one of the most important immunosuppressive cell types in the tumour microenvironment.
- M2 macrophages
-
Alternatively activated macrophages that do not express the typical markers of inflammatory macrophages (M1) and are most commonly found at sites of angiogenesis and tissue regeneration, as well as in the tumour microenvironment. M2 macrophages are weakly pro-inflammatory and mostly anti-inflammatory and immunosuppressive.
- Macropinocytosis
-
A regulated form of endocytosis that mediates the uptake of extracellular fluid and small soluble molecules.
- P2X7B
-
A shorter (364 amino acids long) splice variant of the human P2X purinergic receptor 7 (P2X7) subunit (the full length variant being named P2X7A) that lacks 231 carboxy-terminal residues and bears an insertion of 18 extra amino acids. P2X7B retains cation (Na+, K+ and Ca2+) channel permeability but lacks the ability to activate the large-conductance pore (macropore) associated with P2X7A activation.
- Pyroptosis
-
A form of cell death that is triggered by inflammasome assembly and mediated by caspase 1 or caspase 11, caspase 4 and caspase 5 activation; the process culminates in gasdermin D cleavage and cell lysis. Pyroptosis is thought to be a defensive lytic mechanism to kill infected cells.
- Anaplerotic reactions
-
Biochemical processes that generate intermediates of metabolic pathways, which are often used as building blocks for the synthesis of structural cell components.
- Warburg effect
-
Also known as aerobic glycolysis, an effect in which cells preferentially use glycolytic as opposed to oxidative metabolism to synthesize ATP. Aerobic glycolysis was originally thought to be characteristic of tumours but is now known to be characteristic of every fast-growing tissue.
- Autophagy
-
A self-degradative, highly conserved process by which eukaryotic cells digest damaged organelles, intracellular components and protein aggregates and eliminate intracellular pathogens.
- Invadopodia
-
1–2 μm-long, actin-rich membrane protrusions that infiltrate and degrade the extracellular matrix and are considered a hallmark of tumour cells that are undergoing invasion and dissemination.
- Thrombocytopenia
-
A condition characterized by a pathological decrease of platelets (thrombocytes) in the blood.
- Thrombocytosis
-
A condition characterized by a pathological increase of platelets (thrombocytes) in the blood.
- Non-functional P2X7R
-
(nfP2X7R). An as yet poorly characterized P2X purinergic receptor 7 (P2X7R) variant selectively expressed by some tumour types, which exposes an epitope normally hidden in the canonical P2X7R variant and lacks the ability to activate a large-conductance pore.
- Nanobody
-
A single-domain antibody consisting of the variable domain of heavy chain antibodies naturally occurring in camelids. Nanobodies are potentially very useful because they often bind functional epitopes not accessible to conventional antibodies.
- Atherothrombotic events
-
Events leading to the formation of a thrombus on the surface of pre-existing atherosclerotic lesions (fibrous plaques in blood vessels).
Rights and permissions
About this article
Cite this article
Di Virgilio, F., Sarti, A.C., Falzoni, S. et al. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18, 601–618 (2018). https://doi.org/10.1038/s41568-018-0037-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-018-0037-0
This article is cited by
-
Extracellular vesicles as tools and targets in therapy for diseases
Signal Transduction and Targeted Therapy (2024)
-
P2X7 receptors: a bibliometric review from 2002 to 2023
Purinergic Signalling (2024)
-
Purinergic pathways and their clinical use in the treatment of acute myeloid leukemia
Purinergic Signalling (2024)
-
An emerging master inducer and regulator for epithelial-mesenchymal transition and tumor metastasis: extracellular and intracellular ATP and its molecular functions and therapeutic potential
Cancer Cell International (2023)
-
CD39/CD73/A2AR pathway and cancer immunotherapy
Molecular Cancer (2023)