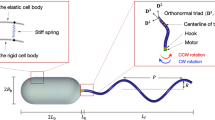
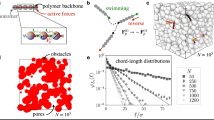
Abstract
The locomotion of swimming bacteria in simple Newtonian fluids can successfully be described within the framework of low-Reynolds-number hydrodynamics1. The presence of polymers in biofluids generally increases the viscosity, which is expected to lead to slower swimming for a constant bacterial motor torque. Surprisingly, however, experiments have shown that bacterial speeds can increase in polymeric fluids2,3,4,5. Whereas, for example, artificial helical microswimmers in shear-thinning fluids6 or swimming Caenorhabditis elegans worms in wet granular media7,8 increase their speeds substantially, swimming Escherichia coli bacteria in polymeric fluids show just a small increase in speed at low polymer concentrations, followed by a decrease at higher concentrations2,4. The mechanisms behind this behaviour are currently unclear, and therefore we perform extensive coarse-grained simulations of a bacterium swimming in explicitly modelled solutions of macromolecular polymers of different lengths and densities. We observe an increase of up to 60% in swimming speed with polymer density and demonstrate that this is due to a non-uniform distribution of polymers in the vicinity of the bacterium, leading to an apparent slip. However, this in itself cannot predict the large increase in swimming velocity: coupling to the chirality of the bacterial flagellum is also necessary.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon request.
References
Lauga, E. Bacterial hydrodynamics. Annu. Rev. Fluid Mech. 48, 105–130 (2016).
Schneider, W. R. & Doetsch, R. N. Effect of viscosity on bacterial motility. J. Bacteriol. 117, 696–701 (1974).
Berg, H. C. & Turner, L. Movement of microorganisms in viscous environments. Nature 278, 349–351 (1979).
Martinez, V. A. et al. Flagellated bacterial motility in polymer solutions. Proc. Natl Acad. Sci. USA 111, 17771–17776 (2014).
Patteson, A. E., Gopinath, A., Goulian, M. & Arratia, P. E. Running and tumbling with E. coli in polymeric solutions. Sci. Rep. 5, 15761 (2015).
Gómez, S., Godínez, F. A., Lauga, E. & Zenit, R. Helical propulsion in shear-thinning fluids. J. Fluid Mech. 812, R3 (2017).
Juarez, G., Lu, K., Sznitman, J. & Arratia, P. E. Motility of small nematodes in wet granular media. Europhys. Lett. 92, 44002 (2010).
Jung, S. Caenorhabditis elegans swimming in a saturated particulate system. Phys. Fluids 22, 031903 (2010).
Azam, F. & Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 5, 782–791 (2007).
McGuckin, M. A., Lindén, S. K., Sutton, P. & Florin, T. H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Man, Y. & Lauga, E. Phase-separation models for swimming enhancement in complex fluids. Phys. Rev. E 92, 023004 (2015).
Magariyama, Y. & Kudo, S. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 83, 733–739 (2002).
Leshansky, A. M. Enhanced low-Reynolds-number propulsion in heterogeneous viscous environments. Phys. Rev. E 80, 051911 (2009).
Praprotnik, M., Delle Site, L. & Kremer, K. Multiscale simulation of soft matter: from scale bridging to adaptive resolution. Annu. Rev. Phys. Chem. 59, 545–571 (2008).
Phan-Thien, N., Tran-Cong, T. & Ramia, M. A boundary-element analysis of flagellar propulsion. J. Fluid Mech. 184, 533–549 (1987).
Watari, N. & Larson, R. G. The hydrodynamics of a run-and-tumble bacterium propelled by polymorphic helical flagella. Biophys. J. 98, 12–17 (2010).
Vogel, R. & Stark, H. Rotation-induced polymorphic transitions in bacterial flagella. Phys. Rev. Lett. 110, 158104 (2013).
Hu, J., Yang, M., Gompper, G. & Winkler, R. G. Modelling the mechanics and hydrodynamics of swimming E. coli. Soft Matter 11, 7867–7876 (2015).
Drescher, K., Dunkel, J., Cisneros, L. H., Ganguly, S. & Goldstein, R. E. Fluid dynamics and noise in bacterial cell–cell and cell–surface scattering. Proc. Natl Acad. Sci. USA 108, 10940–10945 (2011).
Balin, A. K., Zöttl, A., Yeomans, J. M. & Shendruk, T. Biopolymer dynamics driven by helical flagella. Phys. Rev. Fluids 2, 113102 (2017).
de Gennes, P. G. Polymers at an interface; a simplified view. Adv. Colloid Interface Sci. 27, 189–209 (1987).
Tuinier, R., Dhont, J. K. G. & Fan, T.-H. How depletion affects sphere motion through solutions containing macromolecules. Europhys. Lett. 75, 929–935 (2006).
Fan, T.-H., Dhont, J. K. G. & Tuinier, R. Motion of a sphere through a polymer solution. Phys. Rev. E 75, 011803 (2007).
Gray, J. & Hancock, G. J. The propulsion of sea-urchin spermatozoa. J. Exp. Biol. 32, 802–814 (1955).
Chwang, A. T. & Wu, T. Y. A note on the helical movement of micro-organisms. Proc. R. Soc. Lond. B 178, 327–346 (1971).
Rodenborn, B., Chen, C.-H., Swinney, H. L., Liu, B. & Zhang, H. P. Propulsion of microorganisms by a helical flagellum. Proc. Natl Acad. Sci. USA 110, E338–E347 (2013).
Liu, B., Powers, T. R. & Breuer, K. S. Force-free swimming of a model helical flagellum in viscoelastic fluids. Proc. Natl Acad. Sci. USA 108, 19516–19520 (2011).
Spagnolie, S. E., Liu, B. & Powers, T. R. Locomotion of helical bodies in viscoelastic fluids: enhanced swimming at large helical amplitudes. Phys. Rev. Lett. 111, 068101 (2013).
Barr, A. H. Superquadrics and angle-preserving transformations. IEEE Comput. Graph. Appl. 1, 11–23 (1981).
Higdon, J. J. L. A hydrodynamic analysis of flagellar propulsion. J. Fluid Mech. 90, 685–711 (1979).
Reigh, S. Y., Winkler, R. G. & Gompper, G. Synchronization and bundling of anchored bacterial flagella. Soft Matter 8, 4363–4372 (2012).
Allen, M. P. & Tildesley, D. J. Computer Simulation of Liquids (Clarendon, Oxford, England, 1989).
Weeks, J. D., Chandler, D. & Andersen, H. C. Role of repulsive forces in determining the equilibrium structure of simple liquids. J. Chem. Phys. 54, 5237–5247 (1971).
Kapral, R. Multiparticle collision dynamics: simulation of complex systems on mesoscales. Adv. Chem. Phys. 140, 89–146 (2008).
Gompper, G., Ihle, T., Kroll, D. M. & Winkler, R. G. Multi-particle collision dynamics: a particle-based mesoscale simulation approach to the hydrodynamics of complex fluids. Adv. Polym. Sci. 221, 1–87 (2009).
Malevanets, A. & Yeomans, J. M. Dynamics of short polymer chains in solution. Europhys. Lett. 52, 231–237 (2000).
Backer, J. A., Lowe, C. P., Hoefsloot, H. C. J. & Iedema, P. D. Poiseuille flow to measure the viscosity of particle model fluids. J. Chem. Phys. 122, 154503 (2005).
Saramito, P. Complex Fluid Modelling and Algorithms (Springer International, Cham, 2016).
Rubinstein, M. & Colby, R. H. Polymer Physics. (Oxford University Press, Oxford, 2003).
Chattopadhyay, S., Moldovan, R., Yeung, C. & Wu, X. L. Swimming efficiency of bacterium Escherichia coli. Proc. Natl Acad. Sci. USA 103, 13712–13717 (2006).
Kim, S. & Karrila, J. S. Microhydrodynamics - Principles and Selected Applications (Dover, Mineola, 1991).
Acknowledgements
We are grateful to T. Shendruk for helpful discussions and comments on the manuscript. We acknowledge discussions with W. Poon, V. Martinez, A. Morozov, A. Balin and A. Doostmohammadi. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 653284. The authors would like to acknowledge the use of the University of Oxford Advanced Research Computing (ARC) facility in carrying out this work.
Author information
Authors and Affiliations
Contributions
A.Z. and J.M.Y. designed the research. A.Z. developed the MPCD simulation code, performed the simulations, analysed the data and developed the theory. A.Z. and J.M.Y. developed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Physics thanks Alex Leshansky and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–10.
Supplementary Video 1
Dynamics and swimming performance of a bacterium in different fluids. Swimming in a Newtonian fluid in the absence of polymers (top), and in a semi-dilute (density ρ = 0.05, centre) and dense (density ρ = 0.2, bottom) polymer solution consisting of macromolecular semiflexible polymers (length N = 12, stiffness kb = 12kBT). Left: side view; middle: front view; right: back view. The colours of individual polymers are to aid visualization.
Rights and permissions
About this article
Cite this article
Zöttl, A., Yeomans, J.M. Enhanced bacterial swimming speeds in macromolecular polymer solutions. Nat. Phys. 15, 554–558 (2019). https://doi.org/10.1038/s41567-019-0454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-019-0454-3
This article is cited by
-
Chiral active particles are sensitive reporters to environmental geometry
Nature Communications (2024)
-
Orientational dynamics and rheology of active suspensions in weakly viscoelastic flows
Communications Physics (2023)
-
The colloidal nature of complex fluids enhances bacterial motility
Nature (2022)
-
Interaction of microswimmers in viscoelastic liquid crystals
Communications Physics (2022)
-
Mapping Viscoelastic Properties Using Helical Magnetic Nanopropellers
Transactions of the Indian National Academy of Engineering (2021)