Abstract
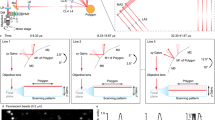
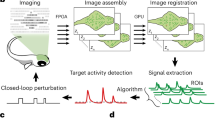
Large-scale imaging of biological dynamics with high spatiotemporal resolution is indispensable to system biology studies. However, conventional microscopes have an inherent compromise between the achievable field of view and spatial resolution due to the space–bandwidth product theorem. In addition, a further challenge is the ability to handle the enormous amount of data generated by a large-scale imaging platform. Here, we break these bottlenecks by proposing the use of a flat–curved–flat imaging strategy, in which the sample plane is magnified onto a large spherical image surface and then seamlessly conjugated to multiple planar sensors. Our real-time, ultra-large-scale, high-resolution (RUSH) imaging platform operates with a 10 × 12 mm2 field of view, a uniform resolution of ~1.20 μm after deconvolution and a data throughput of 5.1 gigapixels per second. We use the RUSH platform to perform video-rate, gigapixel imaging of biological dynamics at centimetre scale and micrometre resolution, including brain-wide structural imaging and functional imaging in awake, behaving mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Anderson, P. W. More is different. Science 177, 93–396 (1972).
Aderem, A. Systems biology: its practice and challenges. Cell 121, 511–513 (2005).
Ji, N., Freeman, J. & Smith, S. L. Technologies for imaging neural activity in large volumes. Nat. Neurosci. 19, 1154–1164 (2016).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Lohmann, A. W., Dorsch, R. G., Mendlovic, D., Zalevsky, Z. & Ferreira, C. Space–bandwidth product of optical signals and systems. J. Opt. Soc. Am. A 13, 470–473 (1996).
Goda, K., Tsia, K. K. & Jalali, B. Serial time-encoded amplified imaging for real-time observation of fast dynamic phenomena. Nature 458, 1145–1149 (2009).
McConnell, G. et al. A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout. eLife 5, e18659 (2016).
Sofroniew, N. J., Flickinger, D., King, J. & Svoboda, K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5, e14472 (2016).
Tian, L. et al. Computational illumination for high-speed in vitro Fourier ptychographic microscopy. Optica 2, 904–911 (2015).
Zheng, G., Horstmeyer, R. & Yang, C. Wide-field, high-resolution Fourier ptychographic microscopy. Nat. Photon. 7, 739–745 (2013).
Tsai, P. S. et al. Ultra-large field-of-view two-photon microscopy. Opt. Express 23, 13833–13847 (2015).
Potsaid, B., Bellouard, Y. & Wen, J. T. Adaptive scanning optical microscope (ASOM): a multidisciplinary optical microscope design for large field of view and high resolution imaging. Opt. Express 13, 6504–6518 (2005).
Werley, C. A., Chien, M. & Cohen, A. E. An ultrawidefield microscope for high-speed fluorescence imaging and targeted optogenetic stimulation. Bio. Opt. Express 8, 5794–5813 (2017).
Stirman, J. N., Smith, I. T., Kudenov, M. W. & Smith, S. L. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 (2016).
Li, A. et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science 330, 1404–1408 (2010).
Economo, M. N. et al. A platform for brain-wide imaging and reconstruction of individual neurons. eLife 5, e10566 (2016).
Luo, W., Zhang, Y., Feizi, A., Göröcs, Z. & Ozcan, A. Pixel super-resolution using wavelength scanning. Light Sci. Appl. 5, e16060 (2016).
Grayson, T. P. Curved focal plane wide-field-of-view telescope design. Proc. SPIE 4849, 269–275 (2002).
Brady, D. J. et al. Multiscale gigapixel photography. Nature 486, 386–389 (2012).
Brady, D. J. et al. Parallel cameras. Optica 5, 127–137 (2018).
Elad, M. & Yacov, H. A fast super-resolution reconstruction algorithm for pure translational motion and common space-invariant blur. IEEE Trans. Image Process 10, 1187–1193 (2001).
Herron, T. J., Lee, P. & Jalife, J. Optical imaging of voltage and calcium in cardiac cells and tissues. Circ. Res. 110, 609–623 (2012).
Ikegaya, Y., Bon-Jego, M. L. & Yuste, R. Large-scale imaging of cortical network activity with calcium indicators. Neurosci. Res. 52, 132–138 (2005).
Gao, P. & Ganguli, S. On simplicity and complexity in the brave new world of large-scale neuroscience. Curr. Opin. Neurobiol. 32, 148–155 (2015).
Kann, O. & Kovács, R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 292, C641–C657 (2007).
Wenzel, M., Hamm, J. P., Peterka, D. S. & Yuste, R. Reliable and elastic propagation of cortical seizures in vivo. Cell Rep. 19, 2681–2693 (2017).
Wang, Y., Goodfellow, M., Taylor, P. N. & Baier, G. Dynamic mechanisms of neocortical focal seizure onset. PLoS Comput. Biol. 10, e1003787 (2014).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Wandell, B. A. What’s in your mind? Nat. Neurosci. 11, 384–385 (2008).
Pantanowitz, L. et al. Review of the current state of whole slide imaging in pathology. J. Pathol. Inform. 2, 36 (2011).
Dittrich, P. S. & Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug Discov. 5, 210–218 (2006).
Lim, D., Chu, K. K. & Mertz, J. Wide-field fluorescence sectioning with hybrid speckle and uniform-illumination microscopy. Opt. Lett. 33, 1819–1821 (2008).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Zhu, G., Howe, J. V., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Lin, X., Wu, J., Zheng, G. & Dai, Q. Camera array based light field microscopy. Biomed. Opt. Express 6, 3179–3189 (2015).
Wilburn, B. et al. High performance imaging using large camera arrays. ACM Trans. Graph. 24, 765–776 (2005).
Gomez, J., Potreau, D., Branka, J. & Raymond, G. Developmental changes in Ca2+ currents from newborn rat cardiomyocytes in primary culture. Pflügers Arch. 428, 241–249 (1994).
Favaron, M. et al. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc. Natl Acad. Sci. USA 85, 7351–7355 (1988).
Chen, T. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Kong, L. et al. Continuous volumetric imaging via an optical phase-locked ultrasound lens. Nat. Methods 12, 759–762 (2015).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Longair, M. H., Baker, D. A. & Armstrong, J. D. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27, 2453–2454 (2011).
Julier, S. J. & Uhlmann, J. K. New extension of the Kalman filter to nonlinear systems. Proc. SPIE 3068, 182–194 (1997).
Acknowledgements
We thank Y. Shu, S. Wang, Y. Li, Z. Guo, B. Zhou, W. Wang and Y. Jia for assistance with sample preparation. We also thank members of Dai’s group for helping with experiments and data analysis: X. Zhang, Y. Zhang, Y. Cheng, K. Liu, C. Zhuang, C. Qiao, Z. Zhao, X. Han, T. Zhou, Y. Zhang, X. Chen, W. Liu, T. Yan, G. Zhang, L. Wang, Y. Ma, X. Hu, J. Hu, T. Zhu, X. Chen, J. Bao and X. Zhang. We acknowledge P. Xi, L. Fang and G. Holtom for their assistance with editing the manuscript. This work is supported by the National Natural Science Foundation of China (61327902, 61671265, 61771287, 61741116, 61722110, 61831014, 31430038 and 81571275) and the Beijing Municipal Science & Technology Commission (Z181100003118014).
Author information
Authors and Affiliations
Contributions
J.F., J.S. and J.W. designed the system architecture. J.F. performed system integration and calibration, aided by F.C. and G.W. J.W. and H.X. conducted performance optimization. H.X. conducted most biological experiments, data capturing and analysis, and Y.S. led the optical and mechanical manufacturing. F.C. and G.W. developed the graphical user interface software. L.C. and G.J. contributed to the optimization of system design. Q.H. designed and conducted experiments on acute human cortical slices from epilepsy patients. T.L. and G.L. contributed to human tissue processing. L.K. conceived and supervised the biological experiments and data analysis, aided by H.X. Z.Z. designed the optical system, aided by J.F., J.S. and J.W. Q.D. conceived and supervised the project, and all authors contributed to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Objective lens design, optical transfer function, point spread function, calcium imaging data.
Supplementary Video 1
Calcium imaging of cardiac cells with periodic behaviour.
Supplementary Video 2
Calcium imaging of cardiac cells with non-periodic dynamics.
Supplementary Video 3
Calcium imaging of neurons.
Supplementary Video 4
Calcium imaging of human cortical slices.
Supplementary Video 5
Calcium imaging of human cortical slices with single-cell resolution.
Supplementary Video 6
Tracking of immune cells.
Supplementary Video 7
Imaging of vascular and calcium signals in awake mice.
Supplementary Video 8
Calcium imaging of neural activity in mice.
Supplementary Video 9
Calcium imaging of cardiac tissue in a microfluidic device.
Rights and permissions
About this article
Cite this article
Fan, J., Suo, J., Wu, J. et al. Video-rate imaging of biological dynamics at centimetre scale and micrometre resolution. Nat. Photonics 13, 809–816 (2019). https://doi.org/10.1038/s41566-019-0474-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-019-0474-7
This article is cited by
-
Surmounting photon limits and motion artifacts for biological dynamics imaging via dual-perspective self-supervised learning
PhotoniX (2024)
-
Light-field flow cytometry for high-resolution, volumetric and multiparametric 3D single-cell analysis
Nature Communications (2024)
-
Random-access wide-field mesoscopy for centimetre-scale imaging of biodynamics with subcellular resolution
Nature Photonics (2024)
-
Reflective multi-immersion microscope objectives inspired by the Schmidt telescope
Nature Biotechnology (2024)
-
Deep learning enables parallel camera with enhanced- resolution and computational zoom imaging
PhotoniX (2023)