Abstract
The initial steps of HIV replication in host cells prime the virus for passage through the nuclear pore and drive the establishment of a productive and irreparable infection1,2. The timely release of the viral genome from the capsid—referred to as uncoating—is emerging as a critical parameter for nuclear import, but the triggers and mechanisms that orchestrate these steps are unknown. Here, we identify β-karyopherin Transportin-1 (TRN-1) as a cellular co-factor of HIV-1 infection, which binds to incoming capsids, triggers their uncoating and promotes viral nuclear import. Depletion of TRN-1, which we characterized by mass spectrometry, significantly reduced the early steps of HIV-1 infection in target cells, including primary CD4+ T cells. TRN-1 bound directly to capsid nanotubes and induced dramatic structural damage, indicating that TRN-1 is necessary and sufficient for uncoating in vitro. Glycine 89 on the capsid protein, which is positioned within a nuclear localization signal in the cyclophilin A-binding loop, is critical for engaging the hydrophobic pocket of TRN-1 at position W730. In addition, TRN-1 promotes the efficient nuclear import of both viral DNA and capsid protein. Our study suggests that TRN-1 mediates the timely release of the HIV-1 genome from the capsid protein shell and efficient viral nuclear import.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
References
Campbell, E. M. & Hope, T. J. HIV-1 capsid: the multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 13, 471–483 (2015).
Ambrose, Z. & Aiken, C. HIV-1 uncoating: connection to nuclear entry and regulation by host proteins. Virology 454–455, 371–379 (2014).
Lahaye, X. et al. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39, 1132–1142 (2013).
Rasaiyaah, J. et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503, 402–405 (2013).
Fernandez, J. et al. Microtubule-associated proteins 1 (MAP1) promote human immunodeficiency virus type I (HIV-1) intracytoplasmic routing to the nucleus. J. Biol. Chem. 290, 4631–4646 (2015).
Twyffels, L., Gueydan, C. & Kruys, V. Transportin-1 and Transportin-2: protein nuclear import and beyond. FEBS Lett. 588, 1857–1868 (2014).
Cohen, S., Au, S. & Pante, N. How viruses access the nucleus. Biochim. Biophys. Acta 1813, 1634–1645 (2011).
Matreyek, K. A. & Engelman, A. Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes. Viruses 5, 2483–2511 (2013).
Cribier, A. et al. Mutations affecting interaction of integrase with TNPO3 do not prevent HIV-1 cDNA nuclear import. Retrovirology 8, 104 (2011).
Ocwieja, K. E. et al. HIV integration targeting: a pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog. 7, e1001313 (2011).
Zhou, L. et al. Transportin 3 promotes a nuclear maturation step required for efficient HIV-1 integration. PLoS Pathog. 7, e1002194 (2011).
Siomi, H. & Dreyfuss, G. A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol. 129, 551–560 (1995).
Lee, B. J. et al. Rules for nuclear localization sequence recognition by karyopherinβ2. Cell 126, 543–558 (2006).
Soniat, M. & Chook, Y. M. Karyopherin-β2 Recognition of a PY-NLS variant that lacks the proline-tyrosine motif. Structure 24, 1802–1809 (2016).
Miyake, Y. et al. Influenza virus uses transportin 1 for vRNP debundling during cell entry. Nat. Microbiol. 4, 578–586 (2019).
Gamble, T. R. et al. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87, 1285–1294 (1996).
Yoo, S. et al. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J. Mol. Biol. 269, 780–795 (1997).
Price, A. J. et al. Active site remodeling switches HIV specificity of antiretroviral TRIMCyp. Nat. Struct. Mol. Biol. 16, 1036–1042 (2009).
Campbell, S. & Vogt, V. M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 69, 6487–6497 (1995).
Li, Y. L. et al. Primate TRIM5 proteins form hexagonal nets on HIV-1 capsids. eLife 5, e16269 (2016).
Francis, A. C., Marin, M., Shi, J., Aiken, C. & Melikyan, G. B. Time-resolved imaging of single HIV-1 uncoating in vitro and in living cells. PLoS Pathog. 12, e1005709 (2016).
Ptak, R. G. et al. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob. Agents Chemother. 52, 1302–1317 (2008).
Arhel, N. Revisiting HIV-1 uncoating. Retrovirology 7, 96 (2010).
Harel, A. & Forbes, D. J. Importin beta: conducting a much larger cellular symphony. Mol. Cell 16, 319–330 (2004).
Greber, U. F. & Fassati, A. Nuclear import of viral DNA genomes. Traffic 4, 136–143 (2003).
Yang, Y., Luban, J. & Diaz-Griffero, F. The fate of HIV-1 capsid: a biochemical assay for HIV-1 uncoating. Methods Mol. Biol. 1087, 29–36 (2014).
Ott, D. E. Cellular proteins detected in HIV-1. Rev. Med. Virol. 18, 159–175 (2008).
Peng, K. et al. Quantitative microscopy of functional HIV post-entry complexes reveals association of replication with the viral capsid. eLife 3, e04114 (2014).
Bejarano, D. A. et al. HIV-1 nuclear import in macrophages is regulated by CPSF6-capsid interactions at the nuclear pore complex. eLife 8, e41800 (2019).
Engelman, A. N. & Singh, P. K. Cellular and molecular mechanisms of HIV-1 integration targeting. Cell. Mol. Life Sci. 75, 2491–2507 (2018).
Fan, X. C. & Steitz, J. A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17, 3448–3460 (1998).
Rankovic, S., Varadarajan, J., Ramalho, R., Aiken, C. & Rousso, I. Reverse transcription mechanically initiates HIV-1 capsid disassembly. J. Virol. 91, e00289-17 (2017).
Arhel, N. J. et al. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 26, 3025–3037 (2007).
Hilditch, L. & Towers, G. J. A model for cofactor use during HIV-1 reverse transcription and nuclear entry. Curr. Opin. Virol. 4, 32–36 (2014).
Marquez, C. L. et al. Kinetics of HIV-1 capsid uncoating revealed by single-molecule analysis. eLife 7, e34772 (2018).
Mallery, D. L. et al. IP6 is an HIV pocket factor that prevents capsid collapse and promotes DNA synthesis. eLife 7, e35335 (2018).
Rawle, D. J. & Harrich, D. Toward the “unravelling” of HIV: host cell factors involved in HIV-1 core uncoating. PLoS Pathog. 14, e1007270 (2018).
Bhargava, A., Lahaye, X. & Manel, N. Let me in: control of HIV nuclear entry at the nuclear envelope. Cytokine Growth Factor Rev. 40, 59–67 (2018).
Stremlau, M. et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004).
Dharan, A. & Campbell, E. M. Role of microtubules and microtubule-associated proteins in HIV-1 infection. J. Virol. 92, e00085-18 (2018).
Chook, Y. M., Jung, A., Rosen, M. K. & Blobel, G. Uncoupling Kapβ2 substrate dissociation and ran binding. Biochemistry 41, 6955–6966 (2002).
Charneau, P. et al. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241, 651–662 (1994).
Strappe, P. M. et al. Identification of unique reciprocal and non reciprocal cross packaging relationships between HIV-1, HIV-2 and SIV reveals an efficient SIV/HIV-2 lentiviral vector system with highly favourable features for in vivo testing and clinical usage. Retrovirology 2, 55 (2005).
De Iaco, A. et al. TNPO3 protects HIV-1 replication from CPSF6-mediated capsid stabilization in the host cell cytoplasm. Retrovirology 10, 20 (2013).
Di Nunzio, F. et al. Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration. PLoS ONE 7, e46037 (2012).
Caron, J. et al. Conical diffraction illumination opens the way for low phototoxicity super-resolution imaging. Cell Adh. Migr. 8, 430–439 (2014).
Schaller, T. et al. Effects of inner nuclear membrane proteins SUN1/UNC-84A and SUN2/UNC-84B on the early steps of HIV-1 infection. J. Virol. 91, e00463-17 (2017).
Kozakov, D. et al. The ClusPro web server for protein–protein docking. Nat. Protoc. 12, 255–278 (2017).
Brooks, B. R. et al. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 4, 187–217 (1983).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Birmanns, S., Rusu, M. & Wriggers, W. Using Sculptor and Situs for simultaneous assembly of atomic components into low-resolution shapes. J. Struct. Biol. 173, 428–435 (2011).
Vizcaino, J. A. et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, 11033 (2016).
Acknowledgements
This work was financed by the Labex EpiGenMed, an ‘Investissements d’avenir’ program, reference ANR-10-LABX-12-01, and by the ATIP-Avenir program (2012–2016), Sidaction (2017–2019), ANRS (Agence Nationale de Recherche sur le Sida), CNRS (Centre National de Recherche Scientifique) and the French Ministry of Higher Education and Research. The atomic force microscopy was financed by the REDSAIM project—Pacte métropolitain d’innovation de Montpellier. We thank the proteomics platform of the Institut Jacques Monod (UMR7592, Paris Diderot University, CNRS) for performing the mass spectrometry, A. Neyret (CEMIPAI) and the Electron Microscopy Platform (COMET) of the Institute for Neurosciences of Montpellier for the electron microscopy, A. Hance (formerly Inserm U941, Hôpital Saint-Louis) for helpful discussions about the project and K. Ford-Powell for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
J.F., A.K.M., S.L., C.C., D.M.P., M.B., S.N. and N.J.A. performed the experiments. T.L., C.G. and L.C. performed the proteomic and structural data analyses. C.H. and M.P. performed the surface plasmon resonance analysis. K.G., D.M. and Y.Y. provided conceptual input. N.J.A. wrote the manuscript with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
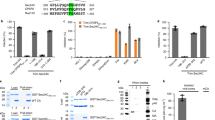
Extended Data Fig. 1 Comparative profiling of TRN-1 knockdown cells.
a, Inhibition of HIV-1 infection by TRN-1 knockdown. P4 TAR-β-gal indicator cells were transduced with EC, shhnRNP E1 or shTRN-1, then infected with VSVG pseudotyped HIV-1. β-gal activity was measured at 2 d.p.i. Results show mean +/− s.d. of multiple replicates from 2 independent experiments. Gels show hnRNP E1 and GAPDH transcript levels following RT-PCR. TRN-1 knockdown is shown in panel b. b, Kap knockdown is specific. HeLa or HEK 293T cells were transduced with the indicated shRNA lentiviral vectors at m.o.i. 50. Results show the mean of triplicates +/- s.e.m. from 3 independent experiments. qPCR results were normalized for housekeeping (HKG) gene transcripts GAPDH or RPL13a. **** p<0.0001, ns=non-significant. Representative western blots from HeLa cells are shown below. c, Effect of Kap knockdown on HIV-1. HeLa cells were treated with specific shRNAs, EC, or not treated (NT), then transduced with a HIV-1-eGFP vector at the indicated m.o.i.s. Infectivity was assessed at 48 hpt by flow cytometry analysis. Results show individual values from 2 independent experiments. d, Kap knockdown does not compromise cell viability. Cell viability was measured by MTT assay at 3 dpt on HeLa cells transduced with the indicated shRNAs. Data show the mean of triplicate samples +/− s.d. and are representative of 3 independent experiments. e, TRN-1 knockdown does not disrupt nuclear pore integrity. Nup214 localization was assessed by confocal fluorescence microscopy in cells treated with EC control, or specific shRNAs against TRN-1 or Nup133, the latter of which was previously shown to disrupt nuclear pore formation 49. Images are representative of 2 independent experiments. Scale bar = 10 µM. f, TRN-1 knockdown cells have a slower proliferation rate. An equal number of HeLa cells treated with either shTRN-1 or EC were counted at 3 dpt. Paired t test was performed on n=16 independent knockdown experiments.
Extended Data Fig. 2 Proteomic characterization of TRN-1 knockdown cells.
a, Proteomic screen of cells depleted of TRN-1. Flowchart of the methodology used to identify proteins that accumulate in the cytosol of TRN-1 knockdown cells. b, The quality of the cytoplasmic (c) and nuclear (n) fractions that were used for mass spectrometry was assessed by western blotting. c, Volcano plot of quantitative mass spectrometry analysis using protein abundance ratios from the shTRN-1 condition compared to the EC (left) and shImpβ-1 (right) conditions. Peptides mixtures were analysed in triplicate. The dashed lines correspond to p-values and Fold Change thresholds of respectively 0.05 and 2. The entire datasets are available from the Pride repository. d, Gene ontology term enrichment analyses of proteins whose abundances are increased in the shTRN-1 condition compared to EC and shImpß-1 conditions. Differential terms (corrected p-value >0.01, Benjamini and Hochberg correction) given by BINGO module and Cytoscape software are represented in yellow.
Extended Data Fig. 3 Characterization of the dependency of HIV-1 infection on TRN-1.
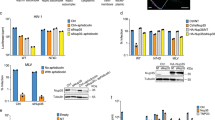
a, HeLa cells depleted of TRN-1 were infected with different HIV-1 reporter viruses or vectors. Infectivity was measured by the percentage of GFP positive cells (flow cytometry) or the luciferase activity (luminescence) at 48 h.p.i. Results show the mean of 3 independent experiments performed in triplicate +/- s.d. Numbers indicate the average fold changes between EC and shTRN-1 samples across 3 independent experiments. b, TRN-1 does not bind HIV-1 integrase. HEK 293T cells were co-transfected with HA-TRN-1, IN-3×FLAG, or empty plasmid pcDNA3.1+. After 24 h, HA or FLAG immunoprecipitation was performed, followed by HA and FLAG western blotting. Blots are representative of 3 independent experiments. c, TRN-1 does not interact with ectopically expressed CA. HEK 293T cells were co-transfected with HA-TRN-1, CA-3×FLAG, or empty plasmid pcDNA3.1+. After 24 h, HA immunoprecipitation was performed, followed by HA- and FLAG- western blotting. The blots are representative of two independent experiments. d, TRN-1 endogenous localization in uninfected cells. HeLa cells were labelled with mouse anti-TRN-1 and rabbit anti-Nup214 antibodies. Images were acquired with a CODIM super-resolution microscope and are representative of 3 independent experiments. C=cytoplasm, N=nucleus. Scale bar = 2 µM. e, TRN-1 and CA co-localize in infected cells. EC or shTRN-1 transduced HeLa cells were infected with HIV-1-VSVG or left uninfected. Duolink labelling was performed at 6 h.p.i. using anti-rabbit TRN-1 and anti-mouse p24. Image analysis was performed in 3D with Imaris on n cells from 3 separate acquisitions (mean values +/- s.e.m.). *** p=0.001, * p=0.198. Results are representative of 2 independent experiments.
Extended Data Fig. 4 The effect of TRN-1 on HIV-1 infection maps to Gly89 on the CypA binding loop of CA.
a, Relative infectivity of the mutants used in Fig. 2d. Results are the mean of 4 (WT) and 2 (mutants) independent experiments performed in triplicate +/- s.e.m. with NL4-3 virus and HIV-1 derived vector. Numbers indicate the average percentages from 3 independent experiments +/- s.d. b, A series of tested HIV-1 mutants did not alter dependency on TRN-1. To examine the role of the central DNA Flap, IN, and CA in TRN-1 dependence of HIV-1 infection, we challenged P4 cells depleted of TRN-1, or TRN-3 as control, with the following HIV-1 mutants: 225T, a central polypurine track (cPPT) mutant that inhibits DNA Flap formation and HIV-1 nuclear import, Q168L, an IN mutant that is deficient for interaction with LEDGF/p75 and TRN-3, and impairs integration, N74D, a point mutation in CA that renders the virus independent of TRN-3. Results show the mean of 2 independent experiments performed in triplicate. p values were ****<0.0001, ***=0.0003, **=0.0036 and ns=0.1635. c, Alignment of the CypA-binding loops of HIV-1, HIV-2, and HIV-2 affine capsid (HIV-ac2). Introducing His-Ala in place of Pro86 in HIV-2 likely repositions Gly87 in the catalytic site and increases binding to CypA 3. d, Introducing His-Ala in place of Pro86 in HIV-2 restores the dependency of HIV-2 for TRN-1. EC and shTRN-1 transduced HeLa cells were infected with HIV-1, HIV-2 or HIV-ac2 viruses at increasing doses. Infectivity was assessed by flow cytometry at 2 d.p.i. Histogram overlays show a representative experiment, while graphs indicate the percentage of GFP-positive cells times the mean fluorescence intensity (MFI) from 3 independent experiments at different m.o.i. ** p=0.0078, * p=0.0156, ns=0.1875.
Extended Data Fig. 5 Recombinant proteins and in vitro binding assays.
a, Coomassie gels of the recombinant CANC and CANC-G89V preparations that were used for the in vitro experiments. The AFM image is representative of 5 independent preparations of in vitro assembled CANC tubes. b, The oligomeric state of CANC in solution was assessed on an ENrich SEC 650 gel-filtration column (Bio-Rad) eluted with 50 mM Tris–HCl pH 8, 100 mM NaCl, at a flow rate of 0.4 ml min−1 at 4 °C. The molecular weight was determined based on a calibration curve obtained using the Gel Filtration Markers Kit for Protein Molecular Weights of 12,400–200,000 Da (Sigma–Aldrich) and dextran blue to assess the column void volume. The apparent mass of CANC was obtained by plotting the partition coefficient Kav against the logarithms of the molecular weights of standard proteins. We estimated this mass to about 60kDa, which is almost two times the theoritical mass of CANC (33kDa). This experiment was repeated twice with similar results. c, Purification of the recombinant TRN-1 stock that was used for Fig. 2f, g and ED Fig. 5e. TRN-1 purification on size-exclusion chromatography Superdex 200 increase 10/300 GL and Coomassie Blue Stained SDS-PAGE of pooled fractions of the SEC column (10 μg of protein loaded on gel). The elution peak corresponds to a monomer (100kDa) and the purity of the protein was estimated to be 95%. d, Coomassie gel of the purified CypA preparation that was used for in vitro experiments. Flow-through from second IMAC shown in lane 3 was used for SPR experiments. e, Kinetic data table obtained from the sensorgram of Fig. 2g using a heterogeneous fitting model. Only the equilibrium constant KD of higher affinity (more than 80% of the total response) is given, ka and kd are rate association and dissociation constants respectively, Rmax the maximum analyte binding capacity, and Chi2 is a measure of the average squared residual (the difference between the experimental data and the fitted curve). This experiment was performed twice with similar results. f, Determination of CypA affinity on WT CANC: SPR fitting curve using steady-state model evaluated from a single cycle kinetic titration of CypA injected at increasing concentrations from 62.5 to 1000 nM on immobilized WT CANC (inset, red curve). Comparatively titration curve of CypA on G89V CANC immobilized (inset, blue). The estimated KD is provided +/- SE. This experiment was performed twice with similar results. g, TRN-1 favours CypA removal from HIV-1 CA. HeLa cells transduced with EC or shTRN-1 were infected with HIV-1-VSVG. Cells were lysed at 4 and 8 h.p.i., capsids were immunoprecipitated and probed with anti-CypA antibody. Results are representative of two independent experiments. h, CypA removal from CA does not rescue dependency of HIV-1 on TRN-1. EC and shTRN-1 HeLa and HEK 293T cells were transduced with shCypA or control vector, or treated with 2 µM Cyclosporin A (CsA), then infected with HIV-1-FLuc virus. Results were normalized for infection in EC cells for each condition. Top graphs show the mean +/- SD of 3 independent experiments, each carried out at different m.o.i. in triplicate. **** p<0.0001. Bottom graphs show the qPCR quantification of TRN-1 and CypA transcripts.
Extended Data Fig. 6 Characterization of the uncoating of HIV-1 by TRN-1.
a, TRN-1 depletion causes HIV-1 capsid to persist in the cytoplasm of infected cells. Hela cells transduced with EC or shTRN-1 were infected with VSVG-pseudotyped HIV-1 in the presence of Raltegravir. Cells were fixed at the indicated times points post-infection. Viral capsid was immunolabelled and 2-4 image stacks were acquired by LSM880 confocal microscope for each time point. CA spots were quantified in 3D using Imaris and are shown as the mean +/- s.e.m. from one experiment, representative of 3 independent experiments. b, TRN-3 depletion does not prevent HIV-1 uncoating. HeLa cells were transduced with shTRN-1 or shTRN-3, then infected with VSVG pseudotyped HIV-1 in the presence of raltegravir. Images show CA labelling in a representative field at 24 h.p.i. Scale bar = 10 µM. Results are representative of two independent experiments. c, Knockdown of TRN-1 does not impede incoming viral trafficking. Hela cells transduced with EC or shTRN-1 were infected with VSV-G pseudotyped HIV-1 and fixed at the indicated time points post-infection. Viral capsid was immunolabelled and 2-4 image stacks were acquired by LSM880 confocal microscope for each time point. The nuclear volume and CA spots within 0.3 μm distance of the nuclear envelope were detected in 3D using Imaris and are shown as the mean +/- s.e.m. from one experiment, representative of 2 independent experiments. Scale bar = 10 µM. d, TEM image of a representative capsid isolated from HIV-1 virions on sucrose cushion, used in Fig. 3b. Scale bar = 100 nm. Results are representative of two independent experiments. e, Coomassie gels of recombinant TRN-1 enrichment used for Fig. 3b. TRN-1 appears as a 102 kDa band (arrows). Quantification is provided at the top of each lane. Other bands may be degradation products of full-length TRN-1. The left-hand blot is a western blot of recombinant wild-type TRN-1 protein. Individual bands from 2 independent preparations were quantified using Image J and the relative abundance of TRN-1 is provided as mean values +/- SD.
Extended Data Fig. 7 Fate of CA and TRN-1 after translocation through the NPC.
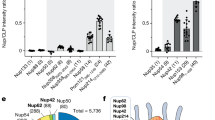
a, Arresting cells in G1/S does not alter HIV-1 dependency on TRN-1. P4 cells were infected with VSVG pseudotyped HIV-1 in the presence of 8 µM Aphidicolin (APH) or DMSO as control. β-gal activity was measured at 2 dpt. Results are the mean of 3 independent experiments performed in triplicate +/- s.e.m. ns=0.5346 and 0.2381 for shTRN-1 and shTNR-3, respectively. Cell cycle arrest in G1/S was confirmed by propidium iodide labelling and flow cytometry (right panel). b, TRN-1 and CA co-localize in the nucleus of target cells at 8 h.p.i. HeLa cells transduced with EC or shTRN-1 eGFP lentiviral vectors, were infected with HIV-1-VSVG or left uninfected. Duolink labelling was performed using anti-rabbit TRN-1 and anti-mouse p24, and image stacks were acquired using a LSM880 confocal microscope. Scale bar = 10 µM. The nuclear volume and CA spots within 0.3 µm distance of the nuclear envelope were detected in 3D using Imaris. Quantification was performed on 3 different stacks and shown as mean +/− SD. Images are representative of 2 independent experiments. c, TRN-1 relocalises to the nucleus in infected cells at 24 h.p.i. HeLa cells were infected with HIV-1-VSVG or left uninfected, and labelled with anti-TRN-1 antibody at 24 h.p.i. Scale bar = 10 µM. Mean grey intensity values in the cytoplasms and nuclei were quantified for each cell using Fiji on n nuclei from 4 separate acquisitions (mean values +/− s.e.m.). **** p<0.0001. Results are representative of 2 independent experiments.
Supplementary information
Supplementary Information
Supplementary Figure legends.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Rights and permissions
About this article
Cite this article
Fernandez, J., Machado, A.K., Lyonnais, S. et al. Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating. Nat Microbiol 4, 1840–1850 (2019). https://doi.org/10.1038/s41564-019-0575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0575-6
This article is cited by
-
The HIV capsid mimics karyopherin engagement of FG-nucleoporins
Nature (2024)
-
Modeling HIV-1 nuclear entry with nucleoporin-gated DNA-origami channels
Nature Structural & Molecular Biology (2023)
-
Nuclear transport proteins: structure, function, and disease relevance
Signal Transduction and Targeted Therapy (2023)
-
Nuclear localization signal peptides enhance genetic transformation of Dunaliella salina
Molecular Biology Reports (2023)
-
HIV-1 capsid variability: viral exploitation and evasion of capsid-binding molecules
Retrovirology (2021)