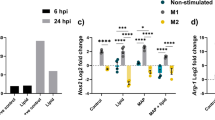
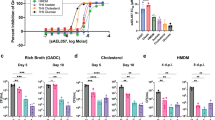
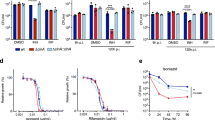
Abstract
Tuberculosis is a significant global health threat, with one-third of the world’s population infected with its causative agent Mycobacterium tuberculosis (Mtb). The emergence of multidrug-resistant (MDR) Mtb that is resistant to the frontline anti-tubercular drugs rifampicin and isoniazid forces treatment with toxic second-line drugs. Currently, ~4% of new and ~21% of previously treated tuberculosis cases are either rifampicin-drug-resistant or MDR Mtb infections1. The specific molecular host–pathogen interactions mediating the rapid worldwide spread of MDR Mtb strains remain poorly understood. W-Beijing Mtb strains are highly prevalent throughout the world and associated with increased drug resistance2. In the early 1990s, closely related MDR W-Beijing Mtb strains (W strains) were identified in large institutional outbreaks in New York City and caused high mortality rates3. The production of interleukin-1β (IL-1β) by macrophages coincides with the shift towards aerobic glycolysis, a metabolic process that mediates protection against drug-susceptible Mtb4. Here, using a collection of MDR W-Mtb strains, we demonstrate that the overexpression of Mtb cell wall lipids, phthiocerol dimycocerosates, bypasses the interleukin 1 receptor, type I (IL-1R1) signalling pathway, instead driving the induction of interferon-β (IFN-β) to reprogram macrophage metabolism. Importantly, Mtb carrying a drug resistance-conferring single nucleotide polymorphism in rpoB (H445Y)5 can modulate host macrophage metabolic reprogramming. These findings transform our mechanistic understanding of how emerging MDR Mtb strains may acquire drug resistance single nucleotide polymorphisms, thereby altering Mtb surface lipid expression and modulating host macrophage metabolic reprogramming.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are available from the authors. DNA sequencing data have been submitted under BioProject ID PRJNA353361. RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database (accession number GSE115495).
Change history
16 October 2018
In the version of this Letter originally published, in Fig. 2d, in the third graph, the label for the y axis was incorrect as ‘TNF-α (pg ml–1)’; it should have read ‘IL-1β (pg ml–1)’. This has now been corrected.
References
Global Tuberculosis Report 2015 WHO/HTM/TB/2015.22 (WHO, 2015).
Bifani, P. J., Mathema, B., Kurepina, N. E. & Kreiswirth, B. N. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10, 45–52 (2002).
Bifani, P. J. et al. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275, 452–457 (1996).
Gleeson, L. E. et al. Cutting Edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J. Immunol. 196, 2444–2449 (2016).
Goldstein, B. P. Resistance to rifampicin: a review. J. Antibiot. 67, 625–630 (2014).
Cooper, A. M. et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178, 2243–2247 (1993).
Flynn, J. L. et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995).
MacMicking, J. D. et al. Indentification of NOS2 as a protective locus against tuberculosis. Proc. Natl Acad. Sci. USA 94, 5243–5248 (1997).
Juffermans, N. P. et al. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182, 902–908 (2000).
Scanga, C. A. et al. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72, 2400–2404 (2004).
Manca, C. et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc. Natl Acad. Sci. USA 98, 5752–5757 (2001).
Desvignes, L., Wolf, A. J. & Ernst, J. D. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J. Immunol. 188, 6205–6215 (2012).
Redford, P. S. et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40, 2200–2210 (2010).
Lachmandas, E. et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 46, 2574–2586 (2016).
Watson, R. O. et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17, 811–819 (2015).
Huang, L., Nazarova, E. V., Tan, S., Liu, Y. & Russell, D. G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 215, 1135–1152 (2018).
O’Neill, L. A. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Noy, T. et al. Central role of pyruvate kinase in carbon co-catabolism of Mycobacterium tuberculosis. J. Biol. Chem. 291, 7060–7069 (2016).
Bisson, G. P. et al. Upregulation of the phthiocerol dimycocerosate biosynthetic pathway by rifampin-resistant, rpoB mutant Mycobacterium tuberculosis. J. Bacteriol. 194, 6441–6452 (2012).
de Knegt, G. J. et al. Rifampicin-induced transcriptome response in rifampicin-resistant Mycobacterium tuberculosis. Tuberculosis 93, 96–101 (2013).
Lahiri, N. et al. Rifampin resistance mutations are associated with broad chemical remodeling of Mycobacterium tuberculosis. J. Biol. Chem. 291, 14248–14256 (2016).
Molodtsov, V., Scharf, N. T., Stefan, M. A., Garcia, G. A. & Murakami, K. S. Structural basis for rifamycin resistance of bacterial RNA polymerase by the three most clinically important RpoB mutations found in Mycobacterium tuberculosis. Mol. Microbiol. 103, 1034–1045 (2017).
Barczak, A. K. et al. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog. 13, e1006363 (2017).
Quigley, J. et al. The cell wall lipid PDIM contributes to phagosomal escape and host cell exit of Mycobacterium tuberculosis. mBio 8, e00148-17 (2017).
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103, (2014).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Hewelt-Belka, W. et al. Untargeted lipidomics reveals differences in the lipid pattern among clinical isolates of Staphylococcus aureus resistant and sensitive to antibiotics. J. Proteome Res. 15, 914–922 (2016).
Kidd, T. J. et al. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol. Med. 9, 430–447 (2017).
Mishra, N. N. et al. Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS ONE 7, e43958 (2012).
Enright, M., Zawadski, P., Pickerill, P. & Dowson, C. G. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae. Microb. Drug Resist. 4, 65–70 (1998).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4 + T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Ford, C. B. et al. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat. Genet. 45, 784–790 (2013).
Young, L., Sung, J., Stacey, G. & Masters, J. R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 5, 929–934 (2010).
Treerat, P. et al. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 10, 1069–1081 (2017).
van Soolingen, D., Hermans, P. W., de Haas, P. E., Soll, D. R. & van Embden, J. D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29, 2578–2586 (1991).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Olson, N. D. et al. Best practices for evaluating single nucleotide variant calling methods for microbial genomics. Front. Genet. 6, 235 (2015).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Hutter, S., Vilella, A. J. & Rozas, J. Genome-wide DNA polymorphism analyses using VariScan. BMC Bioinform. 7, 409 (2006).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Rogerson, B. J. et al. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118, 195–201 (2006).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Sergushichev, A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. Preprint at bioRxiv https://doi.org/10.1101/060012 (2016).
Huang, S. C.-C. et al. Cell-intrinsic lysosomal lipolysis is essential for macrophage alternative activation. Nat. Immunol. 15, 846–855 (2014).
McCarthy Travis, R. et al. Overexpression of Mycobacterium tuberculosis manB, a phosphomannomutase that increases phosphatidylinositol mannoside biosynthesis in Mycobacterium smegmatis and mycobacterial association with human macrophages. Mol. Microbiol. 58, 774–790 (2005).
Torrelles, J. B. et al. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J. Biol. Chem. 279, 41227–41239 (2004).
Flentie, K. N., Stallings, C. L., Turk, J., Minnaard, A. J. & Hsu, F.-F. Characterization of phthiocerol and phthiodiolone dimycocerosate esters of M. tuberculosis by multiple-stage linear ion-trap MS. J. Lipid Res. 57, 142–155 (2016).
Torrelles, J. B. et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J. Biol. Chem. 283, 31417–31428 (2008).
Slayden, R. A. & Barry, C. E. 3rd Analysis of the Lipids of Mycobacterium tuberculosis. Methods Mol. Med. 54, 229–245 (2001).
Torrelles, J. B., Azad, A. K. & Schlesinger, L. S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 177, 1805–1816 (2006).
Acknowledgements
This work was supported by Washington University in St. Louis; NIH grants HL105427, AI123780 and AI111914 to S.A.K. and NIH/NHLBI T32 HL007317-37 to N.C.H. A.S. was supported by the Ministry of Education and Science of the Russian Federation (Project 2.3300.2017/4.6). J.R.-M. was supported by funds from the Department of Medicine, University of Rochester and U19 AI91036. The protein identifications and LC/MS analyses were generated at the Washington University Proteomics Shared Resource (WU-PSR). The WU-PSR is supported by the WU Institute of Clinical and Translational Sciences (grant no. NCATS UL1 TR000448), the WU Mass Spectrometry Research Resource (grant nos. NIGMS P41 GM103422, P60-DK-20579, P30-DK56341) and the Siteman Comprehensive Cancer Center (grant no. NCI P30 CA091842). The authors thank L. Schuettpelz (Washington University in St. Louis), U. Nagarajan (University of North Carolina, Chapel Hill), J.H. Russell (Washington University in St. Louis) and H.W. Virgin IV (Washington University in St. Louis) for generously providing mice, J.M. Scordo (Texas Biomed) and R. Domingo-Gonzalez (Washington University in St. Louis) for technical help and S. Squires and L. Lu (Washington University in St. Louis) for animal breeding. We thank T. Stappenbeck and J. Phillips (Washington University in St. Louis) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
N.C.H., B.M., R.S.K., M.N.A., G.K.A. and S.A.K. conceived the experiments. N.C.H., N.D.M., M.A., B.A.R., J.M., M.B., A.S., E.L., N.K., J.R-M., J.B.T., F.-F.H. and J.-M.B.-L. carried out the experiments. L.C., B.N.K., B.M., S.A.K., M.N.A., R.S.K., J.B.T. provided reagents and Mtb strains. N.C.H., N.D.M., M.A., B.A.R., J.M., M.B., A.S., E.L., N.K., J.R.-M., J.B.T., F.-F.H., M.M., M.N.A., B.M. and S.A.K. conducted the analyses. N.C.H. and S.A.K. wrote the paper. All the authors edited the paper and S.A.K. provided funding and overall project supervision and administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13 and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Howard, N.C., Marin, N.D., Ahmed, M. et al. Mycobacterium tuberculosis carrying a rifampicin drug resistance mutation reprograms macrophage metabolism through cell wall lipid changes. Nat Microbiol 3, 1099–1108 (2018). https://doi.org/10.1038/s41564-018-0245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0245-0
This article is cited by
-
Compensatory evolution in NusG improves fitness of drug-resistant M. tuberculosis
Nature (2024)
-
The pathogenic mechanism of Mycobacterium tuberculosis: implication for new drug development
Molecular Biomedicine (2022)
-
Immunometabolic crosstalk during bacterial infection
Nature Microbiology (2022)
-
Macrophage metabolic reprogramming during chronic lung disease
Mucosal Immunology (2021)
-
Training the trainable cells of the immune system and beyond
Nature Immunology (2020)