Abstract
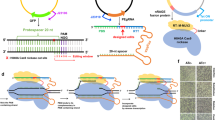
In eukaryotes, the CRISPR–Cas9 system has now been widely used as a revolutionary genome engineering tool1, 2. However, in prokaryotes, the use of nuclease-mediated genome editing tools has been limited to negative selection for the already modified cells because of its lethality3, 4. Here, we report on deaminase-mediated targeted nucleotide editing (Target-AID)5 adopted in Escherichia coli. Cytidine deaminase PmCDA1 fused to the nuclease-deficient CRISPR–Cas9 system achieved specific point mutagenesis at the target sites in E. coli by introducing cytosine mutations without compromising cell growth. The cytosine-to-thymine substitutions were induced mainly within an approximately five-base window of target sequences on the protospacer adjacent motif-distal side, which can be shifted depending on the length of the single guide RNA sequence. Use of a uracil DNA glycosylase inhibitor6 in combination with a degradation tag (LVA tag)7 resulted in a robustly high mutation efficiency, which allowed simultaneous multiplex editing of six different genes. The major multi-copy transposase genes that consist of at least 41 loci were also simultaneously edited by using four target sequences. As this system does not rely on any additional or host-dependent factors, it may be readily applicable to a wide range of bacteria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–827 (2013).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Zhang, Y., Buchholz, F., Muyrers, J. P. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20, 123–128 (1998).
Shimatani, Z. et al. Targeted base editing in rice and tomato using a CRISPR–Cas9 cytidine deaminase fusion. Nat. Biotechnol. 35, 441–443 (2017).
Zhigang, W., Smith, D. G. & Mosbaugh, D. W. Overproduction and characterization of the uracil-DNA glycosylase inhibitor of bacteriophage PBS2. Gene 99, 31–37 (1991).
Andersen, J. B. et al. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64, 2240–2246 (1998).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Costantino, N. & Court, D. L. Enhanced levels of lambda red-mediated recombinants in mismatch repair mutants. Proc. Natl. Acad. Sci. USA 100, 15748–15753 (2003).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Wang, J. et al. An improved recombineering approach by adding RecA to lambda red recombination. Mol. Biotechnol. 32, 43–53 (2006).
Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Bowater, R. & Doherty, A. J. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2, 93–99 (2006).
Cui, L. & Bikard, D. Consequences of Cas9 cleavage in the chromosome of Escherichia coli. Nucleic Acids Res. 44, 4243–4251 (2016).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR–Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Jiang, Y. et al. Multigene editing in the Escherichia coli genome via the CRISPR–Cas9 system. Appl. Environ. Microbiol. 81, 2506–2514 (2015).
Li, Y. et al. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab. Eng. 31, 13–21 (2015).
Pyne, M. E., Moo-Young, M., Chung, D. A. & Chou, C. P. Coupling the CRISPR/Cas9 system with lambda red recombineering enables simplified chromosomal gene replacement in Escherichia coli. Appl. Environ. Microbiol. 81, 5103–5114 (2015).
Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729 (2016).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Yang, L. et al. Engineering and optimising deaminase fusions for genome editing. Nat. Commun. 7, 13330 (2016).
Wang, Y. et al. Bacillus subtilis genome editing using ssDNA with short homology regions. Nucleic Acids Res. 40, e91 (2012).
Warming, S., Costantino, N., Court, D. L., Jenkins, N. A. & Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005).
Jin, D. J., Walter, W. A. & Gross, C. A. Characterization of the termination phenotypes of rifampicin-resistant mutants. J. Mol. Biol. 202, 245–253 (1988).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Rees, H. A. et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun. 8, 15790 (2017).
Ma, Y. et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods 13, 1029–1035 (2016).
Hess, G. T. et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 13, 1036–1042 (2016).
Kim, Y. B. et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 35, 371–376 (2017).
Mitsunobu, H., Teramoto, J., Nishida, K. & Kondo, A. Beyond native Cas9: manipulating genomic information and function. Trends Biotechnol. 35, 983–996 (2017).
Jinek, M. et al. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–822 (2012).
Engler, C., Gruetzner, R., Kandzia, R. & Marillonnet, S. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE 4, e5553 (2009).
Acknowledgements
We would like to thank A. Miyabe and M. Kakimoto for their technical assistance. This work was supported by the Platform Project for Supporting in Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from the Japan Agency for Medical Research and Development (AMED). This work was also partly supported by a Special Coordination Fund for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe) from the Ministry of Education, Culture, Sports and Technology (MEXT) of Japan; the Cross-ministerial Strategic Innovation Promotion Program; JSPS KAKENHI (grant number 26119710, 16K14654); and the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Contributions
S.B. and K.N. performed all of the experiments with help from all authors. S.B., K.N. and H.M. wrote the manuscript with input from all authors. T.A. provided technical advice. K.N. and A.K. conceived the project.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent has been submitted in part entailing the reported approach.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Supplementary Tables 1–7.
Rights and permissions
About this article
Cite this article
Banno, S., Nishida, K., Arazoe, T. et al. Deaminase-mediated multiplex genome editing in Escherichia coli. Nat Microbiol 3, 423–429 (2018). https://doi.org/10.1038/s41564-017-0102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0102-6
This article is cited by
-
Base editor-mediated large-scale screening of functional mutations in bacteria for industrial phenotypes
Science China Life Sciences (2024)
-
Characterization of the AcrIIC1 anti‒CRISPR protein for Cas9‒based genome engineering in E. coli
Communications Biology (2023)
-
Bacterial therapies at the interface of synthetic biology and nanomedicine
Nature Reviews Bioengineering (2023)
-
Prediction of base editor off-targets by deep learning
Nature Communications (2023)
-
Efficient CRISPR-Cas9 based cytosine base editors for phytopathogenic bacteria
Communications Biology (2023)