Abstract
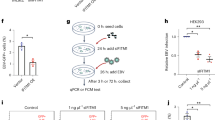
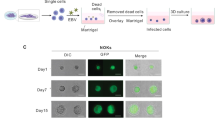
Epstein–Barr virus (EBV) is causally associated with nasopharyngeal carcinoma, 10% of gastric carcinoma and various B cell lymphomas1. EBV infects both B cells and epithelial cells2. Recently, we reported that epidermal growth factor and Neuropilin 1 markedly enhanced EBV entry into nasopharyngeal epithelial cells3. However, knowledge of how EBV infects epithelial cells remains incomplete. To understand the mechanisms through which EBV infects epithelial cells, we integrated microarray and RNA interference screen analyses and found that Ephrin receptor A2 (EphA2) is important for EBV entry into the epithelial cells. EphA2 short interfering RNA knockdown or CRISPR–Cas9 knockout markedly reduced EBV epithelial cell infection, which was mostly restored by EphA2 complementary DNA rescue. EphA2 overexpression increased epithelial cell EBV infection. Soluble EphA2 protein, antibodies against EphA2, soluble EphA2 ligand EphrinA1, or the EphA2 inhibitor 2,5-dimethylpyrrolyl benzoic acid derivative efficiently blocked EBV epithelial cell infection. Mechanistically, EphA2 interacted with EBV entry proteins gH/gL and gB to facilitate EBV internalization and fusion. The EphA2 Ephrin-binding domain and fibronectin type III repeats domain were essential for EphA2-mediated EBV infection, while the intracellular domain was dispensable. This is distinct from Kaposi’s sarcoma-associated herpesvirus infection through EphA24. Taken together, our results identify EphA2 as a critical player for EBV epithelial cell entry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lieberman, P. M. Virology. Epstein–Barr virus turns 50. Science 343, 1323–1325 (2014).
Hutt-Fletcher, L. M. Epstein–Barr virus entry. J. Virol. 81, 7825–7832 (2007).
Wang, H. B. et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 6, 6240 (2015).
Hahn, A. S. et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. Nat. Med. 18, 961–966 (2012).
Thorley-Lawson, D. A., Hawkins, J. B., Tracy, S. I. & Shapiro, M. The pathogenesis of Epstein–Barr virus persistent infection. Curr. Opin. Virol. 3, 227–232 (2013).
Shannon-Lowe, C. & Rowe, M. Epstein Barr virus entry; kissing and conjugation. Curr. Opin. Virol. 4, 78–84 (2014).
Ogembo, J. G. et al. Human complement receptor type 1/CD35 is an Epstein–Barr Virus receptor. Cell Rep. 3, 371–385 (2013).
Wang, X., Kenyon, W. J., Li, Q., Mullberg, J. & Hutt-Fletcher, L. M. Epstein–Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72, 5552–5558 (1998).
Turk, S. M., Jiang, R., Chesnokova, L. S. & Hutt-Fletcher, L. M. Antibodies to gp350/220 enhance the ability of Epstein–Barr virus to infect epithelial cells. J. Virol. 80, 9628–9633 (2006).
Kirschner, A. N., Omerovic, J., Popov, B., Longnecker, R. & Jardetzky, T. S. Soluble Epstein–Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J. Virol. 80, 9444–9454 (2006).
Borza, C. M. & Hutt-Fletcher, L. M. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat. Med. 8, 594–599 (2002).
Chesnokova, L. S. & Hutt-Fletcher, L. M. Fusion of Epstein–Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 85, 13214–13223 (2011).
Chesnokova, L. S., Nishimura, S. L. & Hutt-Fletcher, L. M. Fusion of epithelial cells by Epstein–Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins αvβ6 or αvβ8. Proc. Natl Acad. Sci. USA 106, 20464–20469 (2009).
Xiong, D. et al. Nonmuscle myosin heavy chain IIA mediates Epstein–Barr virus infection of nasopharyngeal epithelial cells. Proc. Natl Acad. Sci. USA 112, 11036–11041 (2015).
Chakraborty, S., Veettil, M. V., Bottero, V. & Chandran, B. Kaposi’s sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. Proc. Natl Acad. Sci. USA 109, E1163–E1172 (2012).
Wykosky, J. & Debinski, W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol. Cancer Res. 6, 1795–1806 (2008).
Hahn, A. S. & Desrosiers, R. C. Binding of the Kaposi’s sarcoma-associated herpesvirus to the ephrin binding surface of the EphA2 receptor and its inhibition by a small molecule. J. Virol. 88, 8724–8734 (2014).
Valencia, S. M. & Hutt-Fletcher, L. M. Important but differential roles for actin in trafficking of Epstein–Barr virus in B cells and epithelial cells. J. Virol. 86, 2–10 (2012).
Garcia, N. J., Chen, J. & Longnecker, R. Modulation of Epstein–Barr virus glycoprotein B (gB) fusion activity by the gB cytoplasmic tail domain. mBio 4, e00571–00512 (2013).
Hafner, C. et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 50, 490–499 (2004).
Pasquale, E. B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008).
Shao, Z., Zhang, W. F., Chen, X. M. & Shang, Z. J. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: correlation with the angiogenesis and clinical outcome. Oral Oncol. 44, 1110–1117 (2008).
Rivera, R. S. et al. Involvement of EphA2 in head and neck squamous cell carcinoma: mRNA expression, loss of heterozygosity and immunohistochemical studies. Oncol. Rep. 19, 1079–1084 (2008).
Huang, J. et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene 33, 2737–2747 (2014).
Tan, P. et al. EphA2 silencing in nasopharyngeal carcinoma leads to decreased proliferation, invasion and increased sensitization to paclitaxel. Oncol. Lett. 4, 429–434 (2012).
Chandran, B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. J. Virol. 84, 2188–2199 (2010).
Breuss, J. M. et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108, 2241–2251 (1995).
Sheppard, D. Functions of pulmonary epithelial integrins: from development to disease. Physiol. Rev. 83, 673–686 (2003).
Ruegg, C. & Mariotti, A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol. Life Sci. 60, 1135–1157 (2003).
Weis, S. M. & Cheresh, D. A. αV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect Med. 1, a006478 (2011).
Song, L. B. et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 66, 6225–6232 (2006).
Yan, M. et al. IKKα restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nat. Commun. 5, 3661 (2014).
Lung, H. L. et al. TSLC1 is a tumor suppressor gene associated with metastasis in nasopharyngeal carcinoma. Cancer Res. 66, 9385–9392 (2006).
Acknowledgements
This study was supported by the National Key R&D Program (2016YFA0502100 and 2017YFA0505600), the National Natural Science Foundation of China (81520108022, 81502374, 81230045, 81372244, 81572600), the Science and Technology project of Guangdong Province (2014B050504004, 2015B050501005), the talent program of Guangdong Province (412022693047) and the Science and Technology project of Guangdong Province (2014B050504004, 2015B050501005). PlasmidspCAGT7 and pT7EMCLuc were kindly provided by Professor Richard Longnecker and Patricia G. Spear (Northwestern University). Plasmid p2670 was generously gifted from Professor Wolfgang Hammerschmidt (Helmholtz Zentrum München). B.E.G. is supported by a Burroughs Wellcome Foundation Career Award in Medical Sciences. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval number RDDB2017000231.
Author information
Authors and Affiliations
Contributions
M.-S.Z., B.Z., and Z.Q.Z. conceived and designed the experiments, provided supervision and wrote the manuscript. H.Z. conceived the experiments. H.Z., Y.L. and H.-B.W. performed, analysed the key experiments and wrote the manuscript. A.Z., M.-L.C., Z.-X.F., J-Y.H. and X.D.D. performed the experiments. Y.Z.Z. Q.Z., S-B.L, D.X. and Y.D. designed and analysed the data. M.-Z.L., A.-J.Z., E.K., B.G. and Y.-M.L. contributed reagents and materials. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–12, Supplementary Table 1
Rights and permissions
About this article
Cite this article
Zhang, H., Li, Y., Wang, HB. et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nat Microbiol 3, 1–8 (2018). https://doi.org/10.1038/s41564-017-0080-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0080-8
This article is cited by
-
Interferon-induced transmembrane protein-1 competitively blocks Ephrin receptor A2-mediated Epstein–Barr virus entry into epithelial cells
Nature Microbiology (2024)
-
Molecular mimicry of host short linear motif-mediated interactions utilised by viruses for entry
Molecular Biology Reports (2023)
-
Interplay between Epstein-Barr virus infection and environmental xenobiotic exposure in cancer
Infectious Agents and Cancer (2021)
-
A potent and protective human neutralizing antibody targeting a novel vulnerable site of Epstein-Barr virus
Nature Communications (2021)
-
Evolutionary dynamics of multidrug resistant Salmonella enterica serovar 4,[5],12:i:- in Australia
Nature Communications (2021)