Abstract
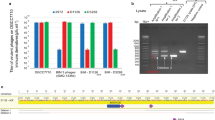
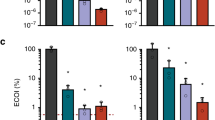
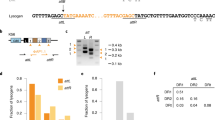
The CRISPR–Cas system owes its utility as a genome-editing tool to its origin as a prokaryotic immune system. The first demonstration of its activity against bacterial viruses (phages) is also the first record of phages evading that immunity1. This evasion can be due to point mutations1, large-scale deletions2, DNA modifications3, or phage-encoded proteins that interfere with the CRISPR–Cas system, known as anti-CRISPRs (Acrs)4. The latter are of biotechnological interest, as Acrs can serve as off switches for CRISPR-based genome editing5. Every Acr characterized to date originated from temperate phages, genomic islands, or prophages4,5,6,7,8, and shared properties with the first Acr discovered. Here, with a phage-oriented approach, we have identified an unrelated Acr in a virulent phage of Streptococcus thermophilus. In challenging a S. thermophilus strain CRISPR-immunized against a set of virulent phages, we found one that evaded the CRISPR-encoded immunity >40,000× more often than the others. Through systematic cloning of its genes, we identified an Acr solely responsible for the abolished immunity. We extended our findings by demonstrating activity in another S. thermophilus strain, against unrelated phages, and in another bacterial genus immunized using the heterologous SpCas9 system favoured for genome editing. This Acr completely abolishes SpCas9-mediated immunity in our assays.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008).
Bryson, A. L. et al. Covalent modification of bacteriophage T4 DNA inhibits CRISPR–Cas9. mBio 6, e00648-15 (2015).
Bondy-Denomy, J., Pawluk, A., Maxwell, K. L. & Davidson, A. R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432 (2013).
Rauch, B. J. et al. Inhibition of CRISPR–Cas9 with bacteriophage proteins. Cell 168, 150–158 (2017).
Pawluk, A., Bondy-Denomy, J., Cheung, V. H. W., Maxwell, K. L. & Davidson, A. R. A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR–Cas system of Pseudomonas aeruginosa. mBio 5, e00896-14 (2014).
Pawluk, A. et al. Inactivation of CRISPR–Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat. Microbiol. 1, 16085 (2016).
Pawluk, A. et al. Naturally occurring off-switches for CRISPR–Cas9. Cell 167, 1829–1838 (2016).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
Makarova, K. S. et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Makarova, K. S., Zhang, F. & Koonin, E. V. SnapShot: class 2 CRISPR–Cas systems. Cell 168, 328–328 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Chowdhury, S. et al. Structure reveals mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. Cell 169, 47–57 (2017).
Maxwell, K. L. et al. The solution structure of an anti-CRISPR protein. Nat. Commun. 7, 13134 (2016).
Dong, D. et al. Structural basis of CRISPR–SpyCas9 inhibition by an anti-CRISPR protein. Nature 546, 436–439 (2017).
Bondy-Denomy, J. et al. Multiple mechanisms for CRISPR–Cas inhibition by anti-CRISPR proteins. Nature 526, 136–139 (2015).
Hynes, A. P., Villion, M. & Moineau, S. Adaptation in bacterial CRISPR–Cas immunity can be driven by defective phages. Nat. Commun. 5, 4399 (2014).
Hynes, A. P., Labrie, S. J. & Moineau, S. Programming native CRISPR arrays for the generation of targeted immunity. mBio 7, e00202-16 (2016).
Hynes, A. P. et al. Detecting natural adaptation of the Streptococcus thermophilus CRISPR–Cas systems in research and classroom settings. Nat. Protoc. 12, 547–565 (2017).
Wei, Y., Chesne, M. T., Terns, R. M. & Terns, M. P. Sequences spanning the leader-repeat junction mediate CRISPR adaptation to phage in Streptococcus thermophilus. Nucleic Acids Res. 43, 1749–1758 (2015).
Magadán, A. H., Dupuis, M.-È., Villion, M. & Moineau, S. Cleavage of phage DNA by the Streptococcus thermophilus CRISPR3-Cas system. PLoS ONE 7, e40913 (2012).
Heler, R. et al. Cas9 specifies functional viral targets during CRISPR–Cas adaptation. Nature 519, 199–202 (2015).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR–Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Lemay, M.-L., Tremblay, D. M. & Moineau, S. Genome engineering of virulent lactococcal phages using CRISPR–Cas9. ACS Synth. Biol. 6, 1351–1358 (2017).
Holo, H. & Nes, I. F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Env. Microbiol. 55, 3119–3123 (1989).
Boisvert, S., Raymond, F., Godzaridis, E., Laviolette, F. & Corbeil, J. Ray Meta: scalable de novo metagenome assembly and profiling. Genome Biol. 13, R122 (2012).
Rombel, I. T., Sykes, K. F., Rayner, S. & Johnston, S. A. ORF-FINDER: a vector for high-throughput gene identification. Gene 282, 33–41 (2002).
Lukashin, A. V. & Borodovsky, M. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26, 1107–1115 (1998).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Alva, V., Nam, S.-Z., Söding, J. & Lupas, A. N. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res 44, W410–W415 (2016).
Drozdetskiy, A., Cole, C., Procter, J. & Barton, G. J. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 43, W389–W394 (2015).
O’Sullivan, D. J. & Klaenhammer, T. R. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 137, 227–231 (1993).
Acknowledgements
The authors thank M.-C. Jodeau and I. Chavichvily for the initial technical work on the efficiency of spacer acquisition in S. thermophilus DGCC7854. A.P.H. is supported by an NSERC Postdoctoral Fellowships award. M.-L.L. is supported by scholarships from the Fonds de Recherche du Québec-Nature et Technologies, Novalait and Op+Lait. S.M. acknowledges funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Program and DuPont. S.M. holds a Tier 1 Canada Research Chair in Bacteriophages.
Author information
Authors and Affiliations
Contributions
A.P.H. and S.M. jointly conceived the study. G.M.R., M.-L.L., P.H., D.A.R., and C.F. contributed technical guidance. A.P.H. designed all experiments performed in this study. A.P.H. and G.M.R. generated all subsequent data presented in the manuscript. M.-L.L. created plasmid constructs for the experiments presented in Fig. 4. A.P.H. wrote the manuscript. A.P.H. generated all associated figures. G.M.R., M.-L.L., P.H., D.A.R., C.F. and S.M commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.P.H., G.M.R., M.-L.L., P.H., D.A.R., C.F. and S.M. are co-inventors on patent(s) or patent application(s) related to CRISPR–Cas systems and their various uses.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figure 1, Supplementary Table 1, Supplementary References.
Rights and permissions
About this article
Cite this article
Hynes, A.P., Rousseau, G.M., Lemay, ML. et al. An anti-CRISPR from a virulent streptococcal phage inhibits Streptococcus pyogenes Cas9. Nat Microbiol 2, 1374–1380 (2017). https://doi.org/10.1038/s41564-017-0004-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0004-7
This article is cited by
-
Inhibitors of bacterial immune systems: discovery, mechanisms and applications
Nature Reviews Genetics (2024)
-
Highly host-linked viromes in the built environment possess habitat-dependent diversity and functions for potential virus-host coevolution
Nature Communications (2023)
-
Phylogenetic Analysis of Anti-CRISPR and Member Addition in the Families
Molecular Biotechnology (2023)
-
KPT330 improves Cas9 precision genome- and base-editing by selectively regulating mRNA nuclear export
Communications Biology (2022)
-
A truncated anti-CRISPR protein prevents spacer acquisition but not interference
Nature Communications (2022)