Abstract
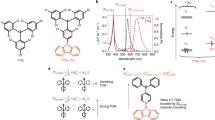
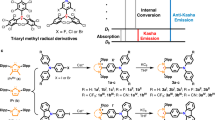
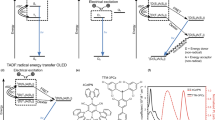
With their unusual electronic structures, organic radical molecules display luminescence properties potentially relevant to lighting applications; yet, their luminescence quantum yield and stability lag behind those of other organic emitters. Here, we designed donor–acceptor neutral radicals based on an electron-poor perchlorotriphenylmethyl or tris(2,4,6-trichlorophenyl)methyl radical moiety combined with different electron-rich groups. Experimental and quantum-chemical studies demonstrate that the molecules do not follow the Aufbau principle: the singly occupied molecular orbital is found to lie below the highest (doubly) occupied molecular orbital. These donor–acceptor radicals have a strong emission yield (up to 54%) and high photostability, with estimated half-lives reaching up to several months under pulsed ultraviolet laser irradiation. Organic light-emitting diodes based on such a radical emitter show deep-red/near-infrared emission with a maximal external quantum efficiency of 5.3%. Our results provide a simple molecular-design strategy for stable, highly luminescent radicals with non-Aufbau electronic structures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the results of this study are available at https://doi.org/10.17863/CAM.41550.
References
Simao, C. et al. Molecular platform for non-volatile memory devices with optical and magnetic responses. Nat. Chem. 3, 359–364 (2011).
Morita, Y. et al. Thermochromism in an organic crystal based on the coexistence of sigma- and pi-dimers. Nat. Mater. 7, 48–51 (2008).
Dhimitruka, I., Bobko, A. A., Eubank, T. D., Komarov, D. A. & Khramtsov, V. V. Phosphonated trityl probes for concurrent in vivo tissue oxygen and pH monitoring using electron paramagnetic resonance-based techniques. J. Am. Chem. Soc. 135, 5904–5910 (2013).
Peng, Q., Obolda, A., Zhang, M. & Li, F. Organic light-emitting diodes using a neutral π radical as emitter: the emission from a doublet. Angew. Chem. Int. Ed. 54, 7091–7095 (2015).
Obolda, A., Ai, X., Zhang, M. & Li, F. Up to 100% formation ratio of doublet exciton in deep-red organic light-emitting diodes based on neutral π-radical. ACS Appl. Mater. Interfaces 8, 35472–35478 (2016).
Ai, X. et al. Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540 (2018).
Hattori, Y., Kusamoto, T. & Nishihara, H. Enhanced luminescent properties of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical by coordination to gold. Angew. Chem. Int. Ed. 54, 3731–3734 (2015).
Hattori, Y., Kusamoto, T. & Nishihara, H. Luminescence, stability, and proton response of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical. Angew. Chem. Int. Ed. 53, 11845–11848 (2014).
Heckmann, A. et al. Highly fluorescent open-shell NIR dyes: the time-dependence of back electron transfer in triarylamine-perchlorotriphenylmethyl radicals. J. Phys. Chem. C 113, 20958–20966 (2009).
Velasco, D. et al. Red organic light-emitting radical adducts of carbazole and tris(2,4,6-trichlorotriphenyl)methyl radical that exhibit high thermal stability and electrochemical amphotericity. J. Org. Chem. 72, 7523–7532 (2007).
Fox, M. A., Gaillard, E. & Chen, C. C. Photochemistry of stable free radicals: the photolysis of perchlorotriphenylmethyl radicals. J. Am. Chem. Soc. 109, 7088–7094 (1987).
Armet, O. et al. Inert carbon free radicals. 8. Polychlorotriphenylmethyl radicals: synthesis, structure, and spin-density distribution. J. Phys. Chem. 91, 5608–5616 (1987).
Ai, X., Chen, Y., Feng, Y. & Li, F. A. Stable room-temperature luminescent biphenylmethyl radical. Angew. Chem. Int. Ed. 57, 2869–2873 (2018).
Hattori, Y., Kusamoto, T. & Nishihara, H. Highly photostable luminescent open-shell (3,5-dihalo-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radicals: significant effects of halogen atoms on their photophysical and photochemical properties. RSC Adv. 5, 64802–64805 (2015).
Kimura, S. et al. Organic radical with two pyridyl groups: high photostability and dual stimuli-responsive properties, with theoretical analyses of photophysical processes. Chem. Sci. 9, 1996–2007 (2018).
Hicks, R. G. What’s new in stable radical chemistry?. Org. Biomol. Chem. 5, 1321–1338 (2007).
Ballester, M., Riera-Figueras, J., Castaner, J., Badfa, C. & Monso, J. M. Inert carbon free radicals. I. Perchlorodiphenylmethyl and perchlorotriphenylmethyl radical series. J. Am. Chem. Soc. 93, 2215–2225 (1971).
Schweyen, P., Brandhorst, K., Wicht, R., Wolfram, B. & Broring, M. The corrole radical. Angew. Chem. Int. Ed. 54, 8213–8216 (2015).
Sun, Z., Ye, Q., Chi, C. & Wu, J. Low band gap polycyclic hydrocarbons: from closed-shell near infrared dyes and semiconductors to open-shell radicals. Chem. Soc. Rev. 41, 7857–7889 (2012).
Bohr, N. Über die Anwendung der Quantentheorie auf den Atombau. I. Die Grundpostulate der Quantentheorie. Z. Phys. 13, 117–165 (1923).
Mallion, R. B. & Rouvray, D. H. Molecular topology and the Aufbau principle. Mol. Phys. 36, 125–128 (1978).
Wang, Y. et al. Radical cation and neutral radical of aza-thia[7]helicene with SOMO−HOMO energy level inversion. J. Am. Chem. Soc. 138, 7298–7304 (2016).
Gryn’ova, G., Coote, M. L. & Corminboeuf, C. Theory and practice of uncommon molecular electronic configurations. WIREs Comput. Mol. Sci. 5, 440–459 (2015).
Franchi, P., Mezzina, E. & Lucarini, M. SOMO–HOMO conversion in distonic radical anions: an experimental test in solution by EPR radical equilibration technique. J. Am. Chem. Soc. 136, 1250–1252 (2014).
Kusamoto, T., Kume, S. & Nishihara, H. Cyclization of TEMPO radicals bound to metalladithiolene induced by SOMO–HOMO energy-level conversion. Angew. Chem. Int. Ed. 49, 529–531 (2010).
Gryn’ova, G., Marshall, D. L., Blanksby, S. J. & Coote, M. L. Switching radical stability by pH-induced orbital conversion. Nat. Chem. 5, 474–481 (2013).
Martin, R. L. Natural transition orbitals. J. Chem. Phys. 118, 4775–4777 (2003).
Komamine, S., Fujitsuka, M., Ito, O. & Itaya, A. Photoinduced electron transfer between C60 and carbazole dimer compounds in a polar solvent. J. Photochem. Photobiol. A 135, 111–117 (2000).
Niu, Y., Peng, Q., Deng, C., Gao, X. & Shuai, Z. Theory of excited state decays and optical spectra: application to polyatomic molecules. J. Phys. Chem. A 114, 7817–7831 (2010).
Hayashi, M., Mebel, A. M., Liang, K. K. & Lin, S. H. Ab Initio calculations of radiationless transitions between excited and ground singlet electronic states of ethylene. J. Chem. Phys. 108, 2044–2055 (1998).
Shizu, K. et al. Enhanced electroluminescence from a thermally activated delayed-fluorescence emitter by suppressing nonradiative decay. Phys. Rev. Appl. 3, 014001–014007 (2015).
Shizu, K. et al. Highly efficient blue electroluminescence using delayed-fluorescence emitters with large overlap density between luminescent and ground states. J. Phys. Chem. C 119, 26283–26289 (2015).
Chen, X.-K., Ravva, M. K., Li, H., Ryno, S. M. & Brédas, J.-L. Effect of molecular packing and charge delocalization on the nonradiative recombination of charge-transfer states in organic solar cells. Adv. Energy Mater. 6, 1601325 (2016).
Sugawara, T., Komatsu, H. & Suzuki, K. Interplay between magnetism and conductivity derived from spin-polarized donor radicals. Chem. Soc. Rev. 40, 3105–3118 (2011).
Kim, D.-H. et al. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nat. Photon. 12, 98–104 (2018).
Ye, H. et al. Near-infrared electroluminescence and low threshold amplified spontaneous emission above 800 nm from a thermally activated delayed fluorescent emitter. Chem. Mater. 30, 6702–6710 (2018).
Körzdörfer, T. & Brédas, J.-L. Organic electronic materials: recent advances in the DFT description of the ground and excited states using tuned range-separated hybrid functionals. Acc. Chem. Res. 47, 3284–3291 (2014).
Sun, H. et al. Impact of dielectric constant on the singlet–triplet gap in thermally activated delayed fluorescence materials. J. Phys. Chem. Lett. 8, 2393–2398 (2017).
Gaussian 09 rev. D01 (Gaussian, Inc., 2009).
Acknowledgements
H.G., Q.P., S.D., X.A., M.Z. and F.L. are grateful for financial support from the National Natural Science Foundation of China (grant nos. 91833304, 51673080 and 11804156), the National Key R&D Programme of China (grant no. 2016YFB0401001), the National Key Basic Research and Development Programme of China (973 programme, grant no. 2015CB655003) and the programme ‘JLUSTIRT’ (grant no. 2019TD-33). Q.P. acknowledges support from the Nanjing Tech Start-up Grant (38274017104). X.-K.C., V.C. and J.-L.B. acknowledge support from the Georgia Institute of Technology, Georgia Research Alliance, Vasser-Woolley Foundation and Kyulux. Q.G., D.C., E.W.E., A.J.G. and R.H.F. would like to thank the EPSRC for funding this work (EP/M01083X/1, EP/M005143/1). Q.G. is also grateful for financial support from the China Scholarship Council and Cambridge Trust. D.C. also acknowledges support from the Royal Society (grant no. UF130278). F.L. is an academic visitor at the Cavendish Laboratory, Cambridge, and is supported by the Talents Cultivation Programme (Jilin University, China).
Author information
Authors and Affiliations
Contributions
H.G., Q.P., S.D., X.A. and M.Z. performed the synthesis and experimental measurements under the supervision of F.L. X.-K.C. carried out the quantum-chemical calculations under the supervision of J.-L.B., and V.C. participated in the discussion of the theoretical calculations. E.W.E. participated in the discussion of the photophysics mechanism, and A.J.G. conducted the transient absorption measurements under the supervision of R.H.F. Q.G. performed the device fabrications and measurements under the supervision of D.C. All authors discussed the results and contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Schemes 1 and 2, Supplementary Figs. 1–13, Supplementary Tables 1–3 and Supplementary refs. 1–29.
Rights and permissions
About this article
Cite this article
Guo, H., Peng, Q., Chen, XK. et al. High stability and luminescence efficiency in donor–acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 18, 977–984 (2019). https://doi.org/10.1038/s41563-019-0433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0433-1
This article is cited by
-
A Stable Open-Shell Conjugated Diradical Polymer with Ultra-High Photothermal Conversion Efficiency for NIR-II Photo-Immunotherapy of Metastatic Tumor
Nano-Micro Letters (2024)
-
Mesitylated trityl radicals, a platform for doublet emission: symmetry breaking, charge-transfer states and conjugated polymers
Nature Communications (2023)
-
Luminescence enhancement by symmetry-breaking in the excited state in radical organic light-emitting diodes
Communications Chemistry (2023)
-
Stable Near-infrared-emitting Radical Nanoparticles for Fluorescence Imaging
Chemical Research in Chinese Universities (2023)
-
Effects of Electron Donating Ability of Substituents and Molecular Conjugation on the Electronic Structures of Organic Radicals
Chemical Research in Chinese Universities (2023)