Abstract
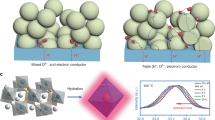
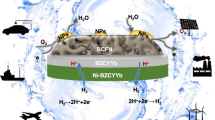
Hydrogen production from water electrolysis is a key enabling energy storage technology for the large-scale deployment of intermittent renewable energy sources. Proton ceramic electrolysers (PCEs) can produce dry pressurized hydrogen directly from steam, avoiding major parts of cost-driving downstream separation and compression. However, the development of PCEs has suffered from limited electrical efficiency due to electronic leakage and poor electrode kinetics. Here, we present the first fully operational BaZrO3-based tubular PCE, with 10 cm2 active area and a hydrogen production rate above 15 Nml min−1. The novel steam anode Ba1−xGd0.8La0.2+xCo2O6−δ exhibits mixed p-type electronic and protonic conduction and low activation energy for water splitting, enabling total polarization resistances below 1 Ω cm2 at 600 °C and Faradaic efficiencies close to 100% at high steam pressures. These tubular PCEs are mechanically robust, tolerate high pressures, allow improved process integration and offer scale-up modularity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Hauch, A., Ebbesen, S. D., Jensen, S. H. & Mogensen, M. Highly efficient high temperature electrolysis. J. Mater. Chem. 18, 2331–2340 (2008).
Laguna-Bercero, M. Recent advances in high temperature electrolysis using solid oxide fuel cells: a review. J. Power Sources 203, 4–16 (2012).
Ebbesen, S. D., Jensen, S. H., Hauch, A. & Mogensen, M. B. High temperature electrolysis in alkaline cells, solid proton conducting cells and solid oxide cells. Chem. Rev. 114, 10697–10734 (2014).
Knibbe, R., Traulsen, M. L., Hauch, A., Ebbesen, S. D. & Mogensen, M. Solid oxide electrolysis cells: degradation at high current densities. J. Electrochem. Soc. 157, B1209–B1217 (2010).
Hauch, A., Jensen, S. H., Ramousse, S. & Mogensen, M. Performance and durability of solid oxide electrolysis cells. J. Electrochem. Soc. 153, A1741–A1747 (2006).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Ishihara, T., Jirathiwathanakul, N. & Zhong, H. Intermediate temperature solid oxide electrolysis cell using LaGaO3 based perovskite electrolyte. Energy Environ. Sci. 3, 665–672 (2010).
Iwahara, H., Uchida, H. & Maeda, N. High temperature fuel and steam electrolysis cells using proton conductive solid electrolytes. J. Power Sources 7, 293–301 (1982).
Norby, T. in Perovskite Oxide for Solid Oxide Fuel Cells (ed. Ishihara, T.) 217–241 (Springer, 2009).
Tong, J., Clark, D., Bernau, L., Sanders, M. & O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 20, 6333–6341 (2010).
Iwahara, H., Yajima, T., Hibino, T., Ozaki, K. & Suzuki, H. Protonic conduction in calcium, strontium and barium zirconates. Solid State Ion. 61, 65–69 (1993).
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Choi, S. et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3, 202–210 (2018).
An, H. et al. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm–2 at 600 °C. Nat. Energy 3, 870–875 (2018).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Morejudo, S. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Malerød-Fjeld, H. et al. Thermo-electrochemical production of compressed hydrogen from methane with near-zero energy loss. Nat. Energy 2, 923–931 (2017).
Babiniec, S. M., Ricote, S. & Sullivan, N. P. Characterization of ionic transport through BaCe0.2Zr0.7Y0.1O3−δ membranes in galvanic and electrolytic operation. Int. J. Hydrogen Energy 40, 9278–9286 (2015).
Bi, L., Shafi, S. P. & Traversa, E. Y-doped BaZrO3 as a chemically stable electrolyte for proton-conducting solid oxide electrolysis cells (SOECs). J. Mater. Chem. A 3, 5815–5819 (2015).
Li, S. & Xie, K. Composite oxygen electrode based on LSCF and BSCF for steam electrolysis in a proton-conducting solid oxide electrolyzer. J. Electrochem. Soc. 160, F224–F233 (2013).
Matsumoto, H., Sakai, T. & Okuyama, Y. Proton-conducting oxide and applications to hydrogen energy devices. Pure Appl. Chem. 85, 427–435 (2012).
Gan, Y. et al. Composite oxygen electrode based on LSCM for steam electrolysis in a proton conducting solid oxide electrolyzer. J. Electrochem. Soc. 159, F763–F767 (2012).
Shang, M., Tong, J. & O’Hayre, R. A promising cathode for intermediate temperature protonic ceramic fuel cells: BaCo0.4Fe0.4Zr0.2O3−δ. RSC Adv. 3, 15769–15775 (2013).
Strandbakke, R. et al. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 278, 120–132 (2015).
Poetzsch, D., Merkle, R. & Maier, J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys. Chem. Chem. Phys. 16, 16446–16453 (2014).
Zohourian, R., Merkle, R. & Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3−δ and comparison to protonic electrolyte materials. Solid State Ion. 299, 64–69 (2017).
Strandbakke, R., Vøllestad, E., Robinson, S. A., Fontaine, M.-L. & Norby, T. Ba0.5Gd0.8La0.7Co2O6−δ infiltrated in porous BaZr0.7Ce0.2Y0.1O3 backbones as electrode material for proton ceramic electrolytes. J. Electrochem. Soc. 164, F196–F202 (2017).
Vollestad, E., Schrade, M., Segalini, J., Strandbakke, R. & Norby, T. Relating defect chemistry and electronic transport in the double perovsksite Ba1−xGd0.8La0.2+xCo2O6−δ(BGLC). J. Mater. Chem. A 5, 15743–15751 (2017).
Brieuc, F., Dezanneau, G., Hayoun, M. & Dammak, H. Proton diffusion mechanisms in the double perovskite cathode material GdBaCo2O5.5: a molecular dynamics study. Solid State Ion. 309, 187–191 (2017).
Mokkelbost, T. et al. High-temperature proton-conducting lanthanum ortho-niobate-based materials. Part II: sintering properties and solubility of alkaline earth oxides. J. Am. Ceram. Soc. 91, 879–886 (2008).
Tong, J., Clark, D., Hoban, M. & O’Hayre, R. Cost-effective solid-state reactive sintering method for high conductivity proton conducting yttrium-doped barium zirconium ceramics. Solid State Ion. 181, 496–503 (2010).
Kwon, O. H. & Choi, G. M. Electrical conductivity of thick film YSZ. Solid State Ion. 177, 3057–3062 (2006).
Timakul, P., Jinawath, S. & Aungkavattana, P. Fabrication of electrolyte materials for solid oxide fuel cells by tape-casting. Ceram. Int. 34, 867–871 (2008).
Kim, S. J., Kim, K. J., Dayaghi, A. M. & Choi, G. M. Polarization and stability of La2NiO4+δ in comparison with La0.6Sr0.4Co0.2Fe0.8O3−δ as air electrode of solid oxide electrolysis cell. Int. J. Hydrogen Energy 41, 14498–14506 (2016).
Jacobsen, T., Chatzichristodoulou, C. & Mogensen, M. B. Fermi potential across working solid oxide cells with zirconia or ceria electrolytes. ECS Trans. 61, 203–214 (2014).
Zhu, H. & Kee, R. J. Membrane polarization in mixed-conducting ceramic fuel cells and electrolyzers. Int. J. Hydrogen Energy 41, 2931–2943 (2016).
Kreuer, K. D. Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003).
Nikodemski, S., Tong, J. & O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ion. 253, 201–210 (2013).
Ricote, S., Manerbino, A., Sullivan, N. P. & Coors, W. G. Preparation of dense mixed electron- and proton-conducting ceramic composite materials using solid-state reactive sintering: BaCe0.8Y0.1M0.1O3−δ–Ce0.8Y0.1M0.1O2−δ (M = Y, Yb, Er, Eu). J. Mater. Sci. 49, 4332–4340 (2014).
Acknowledgements
The work leading to these results has received funding from the Research Council of Norway (grant 236828) and from the European Union’s Seventh Framework Programme (FP7/2007-2013) for the Fuel Cells and Hydrogen Joint Technology Initiative under grant agreement 621244 (‘ELECTRA’) and Fuel Cells and Hydrogen 2 Joint Undertaking under grant agreement 779486 (‘GAMER’). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme, Hydrogen Europe and Hydrogen Europe research.
Author information
Authors and Affiliations
Contributions
E.V., R.S., M.-L.F., J.M.S. and T.N. conceived, designed and supervised the research. D.B. prepared the tubular half cells. M.-L.F. prepared electrodes. E.V. and R.S. developed and synthesized electrode materials, fabricated the electrolysers from tubular half cells and electrode materials and conducted electrochemical characterization. E.V. made the electrochemical model. M.T. conducted stability tests and humidity measurements. D.R.C. performed the TEM work and graphical processing. D.C. and J.M.S. carried out the CFD calculations. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes, Supplementary Figs. 1–15, Supplementary Tables 1–4, Supplementary references 1–14
Rights and permissions
About this article
Cite this article
Vøllestad, E., Strandbakke, R., Tarach, M. et al. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 18, 752–759 (2019). https://doi.org/10.1038/s41563-019-0388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0388-2
This article is cited by
-
Recent progress in oxygen electrodes for protonic ceramic electrochemical cells
Journal of the Korean Ceramic Society (2024)
-
Robust tantalum tuned perovskite oxygen electrode for reversible protonic ceramic electrochemical cells
Rare Metals (2024)
-
Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C
Nature Energy (2023)
-
Enhancing the Faradaic efficiency of solid oxide electrolysis cells: progress and perspective
npj Computational Materials (2023)
-
Oxygen production at intermediate temperatures using Ca2AlMnO5+δ double perovskite-type oxides
Journal of Thermal Analysis and Calorimetry (2023)