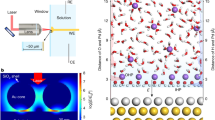
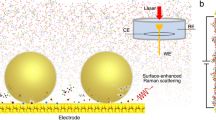
Abstract
Solid/liquid interfaces are ubiquitous in nature and knowledge of their atomic-level structure is essential in elucidating many phenomena in chemistry, physics, materials science and Earth science1. In electrochemistry, in particular, the detailed structure of interfacial water, such as the orientation and hydrogen-bonding network in electric double layers under bias potentials, has a significant impact on the electrochemical performances of electrode materials2,3,4. To elucidate the structures of electric double layers at electrochemical interfaces, we combine in situ Raman spectroscopy and ab initio molecular dynamics and distinguish two structural transitions of interfacial water at electrified Au single-crystal electrode surfaces. Towards negative potentials, the interfacial water molecules evolve from structurally ‘parallel’ to ‘one-H-down’ and then to ‘two-H-down’. Concurrently, the number of hydrogen bonds in the interfacial water also undergoes two transitions. Our findings shed light on the fundamental understanding of electric double layers and electrochemical processes at the interfaces.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Schmickler, W. & Santos, E. Interfacial Electrochemistry (Springer, 2010).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Casalongue, H. S. et al. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 4, 2817 (2013).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Toney, M. F. et al. Voltage-dependent ordering of water molecules at an electrode–electrolyte interface. Nature 368, 444–446 (1994).
Scatena, L. F., Brown, M. G. & Richmond, G. L. Water at hydrophobic surfaces: weak hydrogen bonding and strong orientation effects. Science 292, 908–912 (2001).
Carrasco, J., Hodgson, A. & Michaelides, A. A molecular perspective of water at metal interfaces. Nat. Mater. 11, 667–674 (2012).
Velasco-Velez, J.-J. et al. The structure of interfacial water on gold electrodes studied by X-ray absorption spectroscopy. Science 346, 831–834 (2014).
Guo, J. et al. Real-space imaging of interfacial water with submolecular resolution. Nat. Mater. 13, 184–189 (2014).
Wernet, P. et al. The structure of the first coordination shell in liquid water. Science 304, 995–999 (2004).
Myneni, S. et al. Spectroscopic probing of local hydrogen-bonding structures in liquid water. J. Phys. Condens. Matter 14, L213 (2002).
Ataka, K.-i, Yotsuyanagi, T. & Osawa, M. Potential-dependent reorientation of water molecules at an electrode/electrolyte interface studied by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. 100, 10664–10672 (1996).
Liu, W.-T. & Shen, Y. R. In situ sum-frequency vibrational spectroscopy of electrochemical interfaces with surface plasmon resonance. Proc. Natl Acad. Sci. USA 111, 1293–1297 (2014).
Schultz, Z. D., Shaw, S. K. & Gewirth, A. A. Potential dependent organization of water at the electrified metal–liquid interface. J. Am. Chem. Soc. 127, 15916–15922 (2005).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Chen, Y. X., Zou, S. Z., Huang, K. Q. & Tian, Z. Q. SERS studies of electrode/electrolyte interfacial water part II—librations of water correlated to hydrogen evolution reaction. J. Raman Spectrosc. 29, 749–756 (1998).
Tong, Y., Lapointe, F., Thämer, M., Wolf, M. & Campen, R. K. Hydrophobic water probed experimentally at the gold electrode/aqueous interface. Angew. Chem. Int. Ed. 56, 4211–4214 (2017).
Du, Q., Freysz, E. & Shen, Y. R. Vibrational spectra of water molecules at quartz/water interfaces. Phys. Rev. Lett. 72, 238–241 (1994).
Nihonyanagi, S. et al. Potential-dependent structure of the interfacial water on the gold electrode. Surf. Sci. 573, 11–16 (2004).
Tian, Z.-Q., Ren, B., Chen, Y.-X., Zou, S.-Z. & Mao, B.-W. Probing electrode/electrolyte interfacial structure in the potential region of hydrogen evolution by Raman spectroscopy. J. Chem. Soc. Faraday Trans. 92, 3829–3838 (1996).
Le, J., Iannuzzi, M., Cuesta, A. & Cheng, J. Determining potentials of zero charge of metal electrodes versus the standard hydrogen electrode from density-functional-theory-based molecular dynamics. Phys. Rev. Lett. 119, 016801 (2017).
Cheng, J., Liu, X., VandeVondele, J., Sulpizi, M. & Sprik, M. Redox potentials and acidity constants from density functional theory based molecular dynamics. Acc. Chem. Res. 47, 3522–3529 (2014).
Kolb, D. M. & Schneider, J. Surface reconstruction in electrochemistry: Au(100-(5×20), Au(111)-(1×23) and Au(110)-(1×2). Electrochim. Acta 31, 929–936 (1986).
Noguchi, H., Okada, T. & Uosaki, K. Molecular structure at electrode/electrolyte solution interfaces related to electrocatalysis. Faraday Discuss. 140, 125–137 (2009).
Wasileski, S. A., Koper, M. T. M. & Weaver, M. J. Field-dependent electrode–chemisorbate bonding: sensitivity of vibrational Stark effect and binding energetics to nature of surface coordination. J. Am. Chem. Soc. 124, 2796–2805 (2002).
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys 57, 783–826 (1985).
Scherer, J. R. in Advances in Infrared and Raman Spectroscopy Vol. 5 (eds Clark, R. J. H. & Hester, R. E.) Ch. 3 (Wiley, 1978).
VandeVondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
The authors thank J.W. Yan and S. Liu for helpful discussions. Funding was provdied by the National Natural Science Foundation of China (grants nos. 21373166, 21775127, 21861132015, 21522508, 21521004, 21427813, 21321062, 21621091 and 21533006).
Author information
Authors and Affiliations
Contributions
J.F.L., J.C., C.Y.L. and J.B.L. conceived and designed the project, analysed the results and wrote the manuscript. C.Y.L., Y.H.W., Z.Q.T. and J.F.L. carried out the experiments and analysed the data. J.B.L. and J.C. performed the AIMD calculations. Z.L.Y. and S.C. contributed to FDTD simulations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary materials and methods, Supplementary Figs. 1–8, Supplementary refs. 1–16
Rights and permissions
About this article
Cite this article
Li, CY., Le, JB., Wang, YH. et al. In situ probing electrified interfacial water structures at atomically flat surfaces . Nat. Mater. 18, 697–701 (2019). https://doi.org/10.1038/s41563-019-0356-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0356-x
This article is cited by
-
Impact of the aqueous corrosion induced alteration layer on mechanical properties of pharmaceutical glasses
npj Materials Degradation (2024)
-
Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2
Nature Communications (2024)
-
Ab initio theory of the nonequilibrium adsorption energy
npj Computational Materials (2024)
-
Cu/Mo2C synthesized through Anderson-type polyoxometalates modulate interfacial water structure to achieve hydrogen evolution at high current density
Nano Research (2024)
-
Exploring the Cation Regulation Mechanism for Interfacial Water Involved in the Hydrogen Evolution Reaction by In Situ Raman Spectroscopy
Nano-Micro Letters (2024)