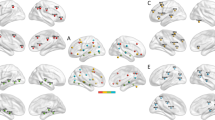
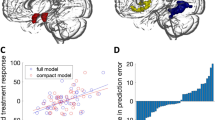
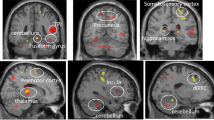
Abstract
The efficacy of antidepressant treatment for depression is controversial due to the only modest superiority demonstrated over placebo. However, neurobiological heterogeneity within depression may limit overall antidepressant efficacy. We sought to identify a neurobiological phenotype responsive to antidepressant treatment by testing pretreatment brain activation during response to, and regulation of, emotional conflict as a moderator of the clinical benefit of the antidepressant sertraline versus placebo. Using neuroimaging data from a large randomized controlled trial, we found widespread moderation of clinical benefits by brain activity during regulation of emotional conflict, in which greater downregulation of conflict-responsive regions predicted better sertraline outcomes. Treatment-predictive machine learning using brain metrics outperformed a model trained on clinical and demographic variables. Our findings demonstrate that antidepressant response is predicted by brain activity underlying a key self-regulatory emotional capacity. Leveraging brain-based measures in psychiatry will forge a path toward better treatment personalization, refined mechanistic insights and improved outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


a, Photo by Nick Huizinga on Unsplash



Similar content being viewed by others
Data availability
All data are publicly available through the NIMH Data Archive (https://nda.nih.gov/edit_collection.html?id=2199).
Code availability
Custom code that supports the findings of this study is available in the Supplementary Software section.
References
Hasin, D. S. et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 75, 336–346 (2018).
Lopez-Munoz, F. & Alamo, C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr. Pharm. Des. 15, 1563–1586 (2009).
Moore, T. J. & Mattison, D. R. Adult utilization of psychiatric drugs and differences by sex, age, and race. JAMA Intern. Med. 177, 274–275 (2017).
Kirsch, I. The Emperor’s New Drugs: Exploding the Antidepressant Myth (Random House, 2009).
Khan, A. & Brown, W. A. Antidepressants versus placebo in major depression: an overview. World Psychiatry 14, 294–300 (2015).
Kirsch, I. et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 5, e45 (2008).
Fournier, J. C. et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303, 47–53 (2010).
Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366 (2018).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23, 28–38 (2017).
Kraemer, H. C. Messages for clinicians: moderators and mediators of treatment outcome in randomized clinical trials. Am. J. Psychiatry 173, 672–679 (2016).
Etkin, A., Buchel, C. & Gross, J. J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693–700 (2015).
Gross, J. J. Handbook of Emotion Regulation (Guilford Press, 2014).
Gyurak, A., Gross, J. J. & Etkin, A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 25, 400–412 (2011).
Xu, M., Xu, G. & Yang, Y. Neural systems underlying emotional and non-emotional interference processing: an ALE meta-analysis of functional neuroimaging studies. Front. Behav. Neurosci. 10, 220 (2016).
Egner, T., Etkin, A., Gale, S. & Hirsch, J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb. Cortex 18, 1475–1484 (2008).
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R. & Hirsch, J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882 (2006).
Maier, M. E. & di Pellegrino, G. Impaired conflict adaptation in an emotional task context following rostral anterior cingulate cortex lesions in humans. J. Cogn. Neurosci. 24, 2070–2079 (2012).
Chechko, N. et al. Brain circuitries involved in emotional interference task in major depression disorder. J. Affect. Disord. 149, 136–145 (2013).
Chechko, N. et al. Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLoS One 4, e5537 (2009).
Etkin, A., Prater, K. E., Hoeft, F., Menon, V. & Schatzberg, A. F. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatry 167, 545–554 (2010).
Etkin, A. & Schatzberg, A. F. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am. J. Psychiatry 168, 968–978 (2011).
Xue, S., Wang, S., Kong, X. & Qiu, J. Abnormal neural basis of emotional conflict control in treatment-resistant depression. Clin. EEG Neurosci. 48, 103–110 (2017).
Widge, A. S. et al. Treating refractory mental illness with closed-loop brain stimulation: progress towards a patient-specific transdiagnostic approach. Exp. Neurol. 287, 461–472 (2017).
Gyurak, A. et al. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biol. Psychiatry 79, 274–281 (2016).
Williams, L. M. et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 40, 2398–2408 (2015).
Etkin, A. et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology 40, 1332–1342 (2015).
Langenecker, S. A. et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol. Psychiatry 62, 1272–1280 (2007).
Pizzagalli, D. A. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36, 183–206 (2011).
Trivedi, M. H. et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J. Psychiatr. Res. 78, 11–23 (2016).
Goldstein-Piekarski, A. N. et al. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry 8, 57 (2018).
Pizzagalli, D. et al. The incremental predictive validity of rostral anterior cingulate cortex activity in relation to symptom improvement in depression: a randomized clinical trial. JAMA Psychiat. 75, 547–554 (2018).
Rush, A. J. et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54, 573–583 (2003).
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R. & Jacobs, G. A. Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, 1983).
Wardenaar, K. J. et al. Development and validation of a 30-item short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ). Psychiatry Res. 179, 101–106 (2010).
Bernstein, D. P. et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136 (1994).
Kerns, J. G. et al. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 (2004).
Korb, A. S., Hunter, A. M., Cook, I. A. & Leuchter, A. F. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin. Neurophysiol. 120, 1313–1319 (2009).
Clayson, P. E. & Larson, M. J. Adaptation to emotional conflict: evidence from a novel face emotion paradigm. PLoS One 8, e75776 (2013).
Tang, D., Hu, L., Chen, A., Clayson, P. E. & Larson, M. J. The neural oscillations of conflict adaptation in the human frontal region. Biol. Psychol. 93, 364–372 (2013).
Larson, M. J., Clawson, A., Clayson, P. E. & Baldwin, S. A. Cognitive conflict adaptation in generalized anxiety disorder. Biol. Psychol. 94, 408–418 (2013).
Suzuki, K. & Shinoda, H. Transition from reactive control to proactive control across conflict adaptation: an sLORETA study. Brain Cogn. 100, 7–14 (2015).
Voigt, J., Carpenter, L. & Leuchter, A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients: a lifetime analysis. PLoS One 12, e0186950 (2017).
Nguyen, K. H. & Gordon, L. G. Cost-effectiveness of repetitive transcranial magnetic stimulation versus antidepressant therapy for treatment-resistant depression. Value Health 18, 597–604 (2015).
O’Reardon, J. P. et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216 (2007).
George, M. S. et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry 67, 507–516 (2010).
Pescosolido, B. A. et al. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am. J. Psychiatry 167, 1321–1330 (2010).
Insel, T. R. & Cuthbert, B. N. Medicine. Brain disorders? Precisely. Science 348, 499–500 (2015).
First, M., Spitzer, R., Gibbon, M. & William, J. Structured Clinical Interview forDSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition (SCID-I/P) (New York State Psychiatric Institute Press, 2002).
Ekman, P. & Friesen, W. V. Pictures of Facial Affect (Consulting Psychologists, 1976).
Gratton, G., Coles, M. G. & Donchin, E. Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen. 121, 480–506 (1992).
Egner, T. & Hirsch, J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 8, 1784–1790 (2005).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Friston, K. J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Fonzo, G. A. et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am. J. Psychiatry 174, 1163–1174 (2017).
Fonzo, G. A. et al. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am. J. Psychiatry 174, 1175–1184 (2017).
Fox, J. Applied Regression Analysis and Generalized Linear Models 2nd edn (Sage Publications, 2008).
Loy, A., Hofmann, H. & Cook, D. Model choice and diagnostics for linear mixed-effects models using statistics on street corners. J. Comput. Graph. Stat. 26, 478–492 (2017).
Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research. R package version 1.8.12. https://CRAN.R-project.org/package=psych (2018).
IBM SPSS Statistics for Macintosh v.21.0 (IBM Corp, 2012).
Huber, P. J. Robust regression: asymptotics, conjectures and monte carlo. Ann. Stat. 1, 799–821 (1973).
Wager, T. D., Keller, M. C., Lacey, S. C. & Jonides, J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26, 99–113 (2005).
R: a language and environment for statistical computing v. 3.2.3 (R Foundation for Statistical Computing, 2015).
Tabelow, K. & Polzehl, J. Statistical parametric maps for functional MRI experiments in R: the package fMRI. 44, 21 (2011).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & R Core Team. nlme: linear and nonlinear mixed effects models. R package version 3.1-141. https://CRAN.R-project.org/package=nlme (2019).
Tipping, M. E. Sparse bayesian learning and the relevance vector machine. J. Mach. Learn. Res. 1, 211–244 (2001).
Tibshirani, R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. B 58, 267–288 (1996).
Cawley, G. C. & Talbot, N. L. Preventing over-fitting during model selection via Bayesian regularisation of the hyper-parameters. J. Mach. Learn. Res. 8, 841–861 (2007).
Wipf, D. P. & Rao, B. D. Sparse bayesian learning for basis selection. IEEE Trans. Signal Process. 52, 2153–2164 (2004).
Zhang, Y. et al. Sparse bayesian classification of EEG for brain-computer interface. IEEE Trans. Neural Netw. Learn. Syst. 27, 2256–2267 (2016).
Cawley, G. C. & Talbot, N. L. Gene selection in cancer classification using sparse logistic regression with Bayesian regularization. Bioinformatics 22, 2348–2355 (2006).
Li, Y., Campbell, C. & Tipping, M. Bayesian automatic relevance determination algorithms for classifying gene expression data. Bioinformatics 18, 1332–1339 (2002).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Soft. 45, 1–67 (2011).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114 (2018).
Choi, E. Y., Yeo, B. T. & Buckner, R. L. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 108, 2242–2263 (2012).
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C. & Yeo, B. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Patenaude, B., Smith, S. M., Kennedy, D. N. & Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56, 907–922 (2011).
Chen, A. C. & Etkin, A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38, 1889–1898 (2013).
Behrens, T. E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757 (2003).
Acknowledgements
The EMBARC study was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers U01MH092221 (M.H.T.) and U01MH092250 (P.J.M., M.M.W.). This work was also funded in part by the Hersh Foundation (M.H.T., principal investigator). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
G.A.F. contributed the analysis and interpretation of the data and the drafting and revision of the manuscript. A.E. contributed to the design of the study, the analysis and interpretation of the data, and the drafting and revision of the manuscript. Y.Z. contributed to the analysis and interpretation of the data and the drafting and revision of the manuscript. W.W. contributed to the analysis and interpretation of the data. C.C., C.C.F., M.K.J. and J.T. contributed to the conduct of the study, analysis and interpretation of the data, and revision of the manuscript. T.D., P.A., M.M., P.J.M. and M.F. contributed to the design and conduct of the study. M.M.W. contributed to the design and conduct of the study, and revision of the manuscript. M.H.T. was the study PI and contributed to study design and funding, conduct of the study, analysis and interpretation of the data, and the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.E. (lifetime disclosure) holds equity in Mindstrong Health and Akili Interactive for unrelated work, has received research funding from the National Institute of Mental Health, Department of Veterans Affairs, Cohen Veterans Bioscience, Brain and Behaviour Research Foundation, Dana Foundation, Brain Resource Inc, and the Stanford Neurosciences Institute, and consulted for Cervel, Takaeda, Posit Science, Acadia, Otsuka, Lundbeck and Janssen. G.A.F. received research support from the National Institute of Mental Health and the Department of Veterans Affairs. T.D.’s research has been funded by NIH, NIMH, NARSAD, TSA, IOCDF, Tufts University, DBDAT and Otsuka Pharmaceuticals, he has received honoraria, consultation fees and/or royalties from the MGH Psychiatry Academy, BrainCells Inc., Clintara, LLC, Inc., Systems Research and Applications Corporation, Boston University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, the Massachusetts Medical Society, Tufts University, NIDA, NIMH, Oxford University Press, Guilford Press and Rutledge. He has also participated in research funded by DARPA, NIH, NIA, AHRQ, PCORI, Janssen Pharmaceuticals, The Forest Research Institute, Shire Development Inc., Medtronic, Cyberonics, Northstar, and Takeda. P.J.M. has received funding from the National Institute of Mental Health, New York State Department of Mental Hygiene, Research Foundation for Mental Hygiene (New York State), Forest Research Laboratories, Sunovion Pharmaceuticals, and Naurex Pharmaceuticals (now Allergan). In the past two years, M.M.W. received funding from the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Sackler Foundation, the Templeton Foundation; and receives royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems. M.F. has received research support from Abbot Laboratories; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Clintara, LLC; Cerecor; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc.; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Tal Medical; Wyeth-Ayerst Laboratories; he has served as advisor or consultant to Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol- Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC Formerly Clinical Trials Solutions, LLC; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; VistaGen; he has received speaking or publishing fees from Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories; he has equity holdings in Compellis and PsyBrain, Inc.; he has a patent for Sequential Parallel Comparison Design (SPCD), which are licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven; and he receives copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd. M.H.T. is or has been an advisor/consultant and received fee from (lifetime disclosure): Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Cerecor, CME Institute of Physicians, Concert Pharmaceuticals, Inc., Eli Lilly & Company, Evotec, Fabre-Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Inc., Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received grants/research support from: Agency for Healthcare Research and Quality (AHRQ), Cyberonics, Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health and National Institute on Drug Abuse. G.A.F., Y.Z., W.W., C.C., C.C.F., M.K., P.A. and J.T. report no competing interests. M.M. has no conflicts of interest with respect to this paper.
Additional information
Peer review information Primary handling editor: Mary Elizabeth Sutherland
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Supplementary Tables 1–10.
Rights and permissions
About this article
Cite this article
Fonzo, G.A., Etkin, A., Zhang, Y. et al. Brain regulation of emotional conflict predicts antidepressant treatment response for depression. Nat Hum Behav 3, 1319–1331 (2019). https://doi.org/10.1038/s41562-019-0732-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-019-0732-1
This article is cited by
-
Major depressive disorder associated alterations in the effective connectivity of the face processing network: a systematic review
Translational Psychiatry (2024)
-
Individualized fMRI connectivity defines signatures of antidepressant and placebo responses in major depression
Molecular Psychiatry (2023)
-
Machine learning-based identification of a psychotherapy-predictive electroencephalographic signature in PTSD
Nature Mental Health (2023)
-
Depression patient-derived cortical neurons reveal potential biomarkers for antidepressant response
Translational Psychiatry (2021)
-
Identification of psychiatric disorder subtypes from functional connectivity patterns in resting-state electroencephalography
Nature Biomedical Engineering (2020)