Abstract
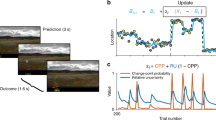
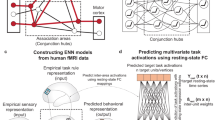
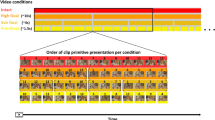
Successful human behaviour depends on the brain’s ability to extract meaningful structure from information streams and make predictions about future events. Individuals can differ markedly in the decision strategies they use to learn the environment’s statistics, yet we have little idea why. Here, we investigate whether the brain networks involved in learning temporal sequences without explicit reward differ depending on the decision strategy that individuals adopt. We demonstrate that individuals alter their decision strategy in response to changes in temporal statistics and engage dissociable circuits: extracting the exact sequence statistics relates to plasticity in motor corticostriatal circuits, while selecting the most probable outcomes relates to plasticity in visual, motivational and executive corticostriatal circuits. Combining graph metrics of functional and structural connectivity, we provide evidence that learning-dependent changes in these circuits predict individual decision strategy. Our findings propose brain plasticity mechanisms that mediate individual ability for interpreting the structure of variable environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Code availability
Custom code used for data analyses is available upon request from the corresponding authors.
Data availability
Behavioural and imaging data in raw and pre-processed format are available upon request from the corresponding authors.
References
Saarinen, J. & Levi, D. M. Perceptual learning in vernier acuity: what is learned? Vision Res. 35, 519–527 (1995).
Christian, J. et al. Socio-cognitive profiles for visual learning in young and older adults. Front. Aging Neurosci. 7, 1–11 (2015).
Siegelman, N., Bogaerts, L., Christiansen, M. H. & Frost, R. Towards a theory of individual differences in statistical learning. Philos. Trans. R. Soc. B 372, 20160059 (2017).
Aslin, R. N. & Newport, E. L. Statistical learning: from acquiring specific items to forming general rules. Curr. Dir. Psychol. Sci. 21, 170–176 (2012).
Acerbi, L., Vijayakumar, S. & Wolpert, D. M. On the origins of suboptimality in human probabilistic inference. PLoS Comput. Biol. 10, e1003661 (2014).
Eckstein, M. P. et al. Rethinking human visual attention: spatial cueing effects and optimality of decisions by honeybees, monkeys and humans. Vision Res. 85, 5–9 (2013).
Murray, R. F., Patel, K. & Yee, A. Posterior probability matching and human perceptual decision making. PLoS Comput. Biol. 11, e1004342 (2015).
Erev, I. & Barron, G. On adaptation, maximization, and reinforcement learning among cognitive strategies. Psychol. Rev. 112, 912–931 (2005).
Shanks, D. R., Tunney, R. J. & McCarthy, J. D. A re-examination of probability matching and rational choice. J. Behav. Decis. Mak. 15, 233–250 (2002).
Wang, R., Shen, Y., Tino, P., Welchman, A. E. & Kourtzi, Z. Learning predictive statistics from temporal sequences: dynamics and strategies. J. Vis. 17, 1 (2017).
Schulze, C., van Ravenzwaaij, D. & Newell, B. R. Of matchers and maximizers: how competition shapes choice under risk and uncertainty. Cogn. Psychol. 78, 78–98 (2015).
Herrnstein, R. J. Relative and absolute strength of response as a function of frequency of reinforcement. J. Exp. Anal. Behav. 4, 267–272 (1961).
Wang, R., Shen, Y., Tino, P., Welchman, A. & Kourtzi, Z. Learning predictive statistics: strategies and brain mechanisms. J. Neurosci. 37, 8412–8427 (2017).
Gheysen, F., Van Opstal, F., Roggeman, C., Van Waelvelde, H. & Fias, W. The neural basis of implicit perceptual sequence learning. Front. Hum. Neurosci. 5, 137 (2011).
Stillman, C. M. et al. Caudate resting connectivity predicts implicit probabilistic sequence learning. Brain Connect. 3, 601–610 (2013).
Turk-Browne, N. B., Scholl, B. J., Chun, M. M. & Johnson, M. K. Neural evidence of statistical learning: efficient detection of visual regularities without awareness. J. Cogn. Neurosci. 21, 1934–1945 (2009).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007).
Deco, G. & Corbetta, M. The dynamical balance of the brain at rest. Neurosci. 17, 107–123 (2011).
Kelly, C. & Castellanos, F. X. Strengthening connections: functional connectivity and brain plasticity. Neuropsychol. Rev. 24, 63–76 (2014).
Sampaio-Baptista, C. & Johansen-Berg, H. White matter plasticity in the adult brain. Neuron 96, 1239–1251 (2017).
Behrens, T. E. J. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757 (2003).
Román, F. J. et al. Enhanced structural connectivity within a brain sub-network supporting working memory and engagement processes after cognitive training. Neurobiol. Learn. Mem. 141, 33–43 (2017).
Heitger, M. H. et al. Motor learning-induced changes in functional brain connectivity as revealed by means of graph-theoretical network analysis. Neuroimage 61, 633–650 (2012).
Farrar, D. & Glauber, R. Multicollinearity in regression analysis: the problem revisited. Rev. Econ. Stat. 49, 92–107 (1967).
Seger, C. A. in The Basal Ganglia IX (eds Groenewegen, H., Voorn, P., Berendse, H., Mulder, A. & Cools, A.) 25–39 (Springer, New York, 2009)..
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289 (2002).
Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Van Dijk, K. R. A. et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 103, 297–321 (2010).
Van den Heuvel, M. P. & Hulshoff Pol, H. E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534 (2010).
Di Martino, A. et al. Functional connectivity of human striatum: a resting state fMRI study. Cereb. Cortex 18, 2735–2747 (2008).
Pauli, W. M., O’Reilly, R. C., Yarkoni, T. & Wager, T. D. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl Acad. Sci. USA 113, 1907–1912 (2016).
Lehéricy, S. et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol. 55, 522–529 (2004).
Draganski, B. et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J. Neurosci. 28, 7143–7152 (2008).
Balleine, B. W. & O’Doherty, J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010).
Piray, P., Toni, I. & Cools, R. Human choice strategy varies with anatomical projections from ventromedial prefrontal cortex to medial striatum. J. Neurosci. 36, 2857–2867 (2016).
McNamee, D., Liljeholm, M., Zika, O. & O’Doherty, J. P. Characterizing the associative content of brain structures involved in habitual and goal-directed actions in humans: a multivariate fMRI study. J. Neurosci. 35, 3764–3771 (2015).
Heekeren, H. R., Marrett, S. & Ungerleider, L. G. The neural systems that mediate human perceptual decision making. Nat. Rev. Neurosci. 9, 467–479 (2008).
Ahissar, M. & Hochstein, S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn. Sci. 8, 457–464 (2004).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013).
Blondel, V. D., Guillaume, J. L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Watts, D. J. & Strogatz, S. H. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442 (1998).
McIntosh, A. R. & Lobaugh, N. J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage 23, 250–263 (2004).
Whitaker, K. J. et al. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc. Natl Acad. Sci. USA 113, 201601745 (2016).
Vértes, P. E. et al. Gene transcription profiles associated with inter-modular hubs and connection distance in human functional magnetic resonance imaging networks. Philos. Trans. R. Soc. Lond. B 371, 735–769 (2016).
Levy, D. J. & Glimcher, P. W. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. 22, 1027–1038 (2012).
Ridderinkhof, K. R., van den Wildenberg, W. P., Segalowitz, S. J. & Carter, C. S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 56, 129–140 (2004).
D’Ardenne, K. et al. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc. Natl Acad. Sci. USA 109, 19900–19909 (2012).
Lewis, C. M., Baldassarre, A., Committeri, G., Romani, G. L. & Corbetta, M. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl Acad. Sci. USA 106, 17558–17563 (2009).
Ventura-Campos, N. et al. Spontaneous brain activity predicts learning ability of foreign sounds. J. Neurosci. 33, 9295–9305 (2013).
Ma, L., Narayana, S., Robin, D. A., Fox, P. T. & Xiong, J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage 58, 226–233 (2011).
Albert, N. B., Robertson, E. M. & Miall, R. C. The resting human brain and motor learning. Curr. Biol. 19, 1023–1027 (2009).
Robbins, T. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos. Trans. R. Soc. B 362, 917–932 (2007).
Sami, S. & Miall, R. C. Graph network analysis of immediate motor-learning induced changes in resting state BOLD. Front. Hum. Neurosci. 7, 1–14 (2013).
Campbell, K. L. et al. Robust resilience of the frontotemporal syntax system to aging. J. Neurosci. 36, 5214–5227 (2016).
Pernet, C. R., Wilcox, R. & Rousselet, G. A. Robust correlation analyses: false positive and power validation using a new open source MATLAB toolbox. Front. Psychol. 3, 606 (2013).
Benjamini, Y. & Yekutieli, D. False discovery rate-adjusted multiple confidence intervals for selected parameters. J. Am. Stat. Assoc. 100, 71–93 (2005).
Krishnan, A., Williams, L. J., McIntosh, A. R. & Abdi, H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage 56, 455–475 (2011).
Sporns, O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 23, 162–171 (2013).
Milan, L. & Whittaker, J. Application of the parametric bootstrap to models that incorporate a singular value decomposition. Appl. Stat. 44, 31–49 (1995).
Acknowledgements
We thank: C. di Bernardi Luft for helping with data collection; the CamGrid team; M. L. Kringelbach, H. M. Fernandes and T. J. Van Hartevelt for help with the DTI analyses; G. Deco for helpful discussions; and H. Johansen-Berg and G. Williams for help with optimizing the DTI sequences and helpful discussions. This work was supported by grants to Z.K. from the Biotechnology and Biological Sciences Research Council (H012508 and BB/P021255/1), Leverhulme Trust (RF-2011-378), Alan Turing Institute (TU/B/000095), Wellcome Trust (205067/Z/16/Z) and (European Community’s) Seventh Framework Programme (FP7/2007-2013) under agreement PITN-GA-2011-290011; A.E.W. from the Wellcome Trust (095183/Z/10/Z) and (European Community’s) Seventh Framework Programme (FP7/2007–2013) under agreement PITN-GA-2012–316746; P.T. from the Engineering and Physical Sciences Research Council (EP/L000296/1); and P.E.V. from the MRC (MR/K020706/1). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.T., A.E.W. and Z.K. designed the research. V.M.K., J.G. and R.W. performed the research. V.M.K., J.G., P.E.V., R.W., Y.S. and P.T. contributed analytical tools. V.M.K. and J.G. analysed the data. All authors co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary References, Supplementary Tables 1–7, Supplementary Figures 1–7
Supplementary Video 1
Resting state graph 3D movie: 3D movie depicting the resting-state graph overlaid on the MNI brain template. The graph is created based on the AAL parcellation (90 areas excluding cerebellum and vermis) and displayed at 5% density for visualization purposes. The thickness of the graph edges is proportional to the average functional connectivity. The selected nodes are coloured to represent regions within known cortico-striatal circuits: caudate and putamen (magenta), right MFG and left IFG (red), postcentral gyrus (cyan), calcarine sulcus (blue) and ACC (yellow).
Supplementary Video 2
DTI graph 3D movie: 3D movie depicting the DTI graph overlaid on the MNI brain template. The graph is created based on the AAL parcellation (90 areas excluding cerebellum and vermis) and displayed at 5% density for visualization purposes. The thickness of the graph edges is proportional to the average structural connectivity. The selected nodes are coloured to represent regions within known cortico-striatal circuits: caudate and putamen (magenta), right MFG and left IFG (red), postcentral gyrus (cyan), calcarine sulcus (blue) and ACC (yellow).
Rights and permissions
About this article
Cite this article
Karlaftis, V.M., Giorgio, J., Vértes, P.E. et al. Multimodal imaging of brain connectivity reveals predictors of individual decision strategy in statistical learning. Nat Hum Behav 3, 297–307 (2019). https://doi.org/10.1038/s41562-018-0503-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0503-4