Abstract
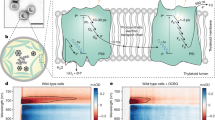
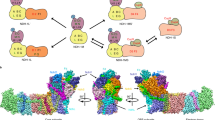
Photosynthetically produced hydrogen is an attractive, sustainable fuel. Semiartificial in vitro techniques have been successfully implemented in which hydrogenases were attached to isolated photosystems for hydrogen production. However, in vitro systems are in general short lived as metabolic processes that support self-repair and maintenance are missing. So far, photosystem–hydrogenase fusions have been tested in vitro only. Here, we report photosynthetic hydrogen production using a photosystem I–hydrogenase fusion in vivo. The NiFe-hydrogenase HoxYH of the cyanobacterium Synechocstis sp. PCC 6803 was fused to its photosystem I subunit PsaD in close proximity to the 4Fe4S cluster FB, which ordinarily donates electrons to ferredoxin. The resultant psaD-hoxYH mutant grows photoautotrophically, achieves a high concentration of photosynthetically produced hydrogen of 500 μM under anaerobic conditions in the light and does not take up the generated hydrogen. Our data indicate that photosynthetic hydrogen production in psaD-hoxYH is most likely based on both oxygenic and anoxygenic photosynthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gutekunst, K. et al. The bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803 is reduced by flavodoxin and ferredoxin and is essential under mixotrophic, nitrate-limiting conditions. J. Biol. Chem. 289, 1930–1937 (2014).
Gutthann, F., Egert, M., Marques, A. & Appel, J. Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 1767, 161–169 (2007).
McIntosh, C. L., Germer, F., Schulz, R., Appel, J. & Jones, A. K. The [NiFe]-hydrogenase of the cyanobacterium Synechocystis sp. PCC 6803 works bidirectionally with a bias to H2 production. J. Am. Chem. Soc. 133, 11308–11319 (2011).
Cournac, L., Guedeney, G., Peltier, G. & Vignais, P. M. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J. Bacteriol. 186, 1737–1746 (2004).
Peden, E. A. et al. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii. J. Biol. Chem. 288, 35192–35209 (2013).
Sawyer, A. & Winkler, M. Evolution of Chlamydomonas reinhardtii ferredoxins and their interactions with [FeFe]-hydrogenases. Photosynth. Res. 134, 307–316 (2017).
Dutta, I. & Vermaas, W. F. J. The electron transfer pathway upon H2 oxidation by the NiFe bidirectional hydrogenase of Synechocystis sp. PCC 6803 in the light shares components with the photosynthetic electron transfer chain in thylakoid membranes. Int. J. Hydrogen Energy 41, 11949–11959 (2016).
Appel, J., Phunpruch, S., Steinmüller, K. & Schulz, R. The bidierctional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch. Microbiol. 173, 333–338 (2000).
Nagy, V. et al. Water-splitting-based, sustainable and efficient H2 production in green algae as achieved by substrate limitation of the Calvin–Benson–Bassham cycle. Biotechnol. Biofuels 11, 1–16 (2018).
Melis, A., Zhang, L., Forestier, M., Ghirardi, M. L. & Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green AlgaChlamydomonas reinhardtii. Plant Physiol. 122, 127–136 (2000).
Ducat, D. C., Sachdeva, G. & Silver, P. A. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc. Natl Acad. Sci. USA 108, 3941–3946 (2011).
Reisner, E., Powell, D. J., Cavazza, C., Fontecilla-Camps, J. C. & Armstrong, F. A. Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles. J. Am. Chem. Soc. 131, 18457–18466 (2009).
Wilker, M. B. et al. Electron transfer kinetics in CdS nanorod–[FeFe]-hydrogenase complexes and implications for photochemical H2 generation. J. Am. Chem. Soc. 136, 4316–4324 (2014).
Hambourger, M. et al. [FeFe]-hydrogenase-catalyzed H2 production in a photoelectrochemical biofuel cell. J. Am. Chem. Soc. 130, 2015–2022 (2008).
Sokol, K. P. et al. Bias-free photoelectrochemical water splitting with photosystem II on a dye-sensitized photoanode wired to hydrogenase. Nat. Energy 3, 944–951 (2018).
Mersch, D. et al. Wiring of photosystem II to hydrogenase for photoelectrochemical water splitting. J. Am. Chem. Soc. 137, 8541–8549 (2015).
Yacoby, I. et al. Photosynthetic electron partitioning between [FeFe]-hydrogenase and ferredoxin:NADP+-oxidoreductase (FNR) enzymes in vitro. Proc. Natl Acad. Sci. USA 108, 9396–9401 (2011).
Lubner, C. E. et al. Solar hydrogen-producing bionanodevice outperforms natural photosynthesis. Proc. Natl Acad. Sci. USA 108, 20988–20991 (2011).
Ihara, M. et al. Light-driven hydrogen production by a hybrid complex of a [NiFe]-hydrogenase and the cyanobacterial photosystem I. Photochem. Photobiol. 82, 676–682 (2006).
Krassen, H. et al. Photosynthetic hydrogen production by a hybrid complex of photosystem I and [NiFe]-hydrogenase. ACS Nano 3, 4055–4061 (2009).
Lubner, C. E., Grimme, R., Bryant, D. A. & Golbeck, J. H. Wiring photosystem I for direct solar hydrogen production. Biochemistry 49, 404–414 (2010).
Utschig, L. M., Soltau, S. R. & Tiede, D. M. Light-driven hydrogen production from photosystem I-catalyst hybrids. Curr. Opin. Chem. Biol. 25, 1–8 (2015).
Ihara, M., Nakamoto, H., Kamachi, T., Okura, I. & Maedal, M. Photoinduced hydrogen production by direct electron transfer from photosystem I cross-linked with cytochrome c 3 to [NiFe]-hydrogenase. Photochem. Photobiol. 82, 1677–1685 (2006).
Aubert-Jousset, E. C., Cano M., Guedeney, G., Richaud, P. & Cournac, L. Role of HoxE subunit in Synechocystis PCC6803 hydrogenase. FEBS J. 4035–4043 (2011).
Schmitz, O. et al. Molecular biological analysis of a bidirectional hydrogenase from cyanobacteria. Eur. J. Biochem. 233, 266–276 (1995).
Serebryakova, L. T. & Sheremetieva, M. E. Characterization of catalytic properties of hydrogenase isolated from the unicellular cyanobacterium Gloeocapsa alpicola CALU 743. Biochem. (Mosc.) 71, 1370–1376 (2006).
Page, C. C., Moser, C. C., Chen, X. & Dutton, P. L. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature 402, 47–52 (1999).
Grotjohann, I. & Fromme, P. Structure of cyanobacterial photosystem I. Photosynth. Res. 85, 51–72 (2005).
Kubota-Kawai, H. et al. X-ray structure of an asymmetrical trimeric ferredoxin–photosystem I complex. Nat. Plants 4, 218–224 (2018).
Fromme, P., Bottin, H., Krauss, N. & Sétif, P. Crystallization and electron paramagnetic resonance characterization of the complex of photosystem I with its natural electron acceptor ferredoxin. Biophys. J. 83, 1760–1773 (2002).
Fromme, P., Jordan, P. & Krauß, N. Structure of photosystem I. Biochim. Biophys. Acta 1507, 5–31 (2001).
Jordan, P. et al. Three-dimensional structure of cyanobbacterial photosystem I at 2.5 Å resolution. Nature 441, 909–917 (2001).
Shomura, Y. et al. Structural basis of the redox switches in the NAD-reducing soluble [NiFe]-hydrogenase. Science 357, 928–932 (2017).
Chitnis, V. P., Ke, A. & Chitnis, P. R. The PsaD subunit of photosystem I (mutations in the basic domain reduce the level of PsaD in the membranes). Plant Physiol. 115, 1699–1705 (1997).
Schuller, J. M. et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 363, 257–260 (2019).
Kothari, A., Potrafka, R. & Garcia-Pichel, F. Diversity in hydrogen evolution from bidirectional hydrogenases in cyanobacteria from terrestrial, freshwater and marine intertidal environments. J. Biotechnol. 162, 105–114 (2012).
Iwuchukwu, I. J. et al. Self-organized photosynthetic nanoparticle for cell-free hydrogen production. Nat. Nanotechnol. 5, 73–79 (2009).
Millsaps, J. F., Bruce, B. D., Lee, J. W. & Greenbaum, E. Nanoscale photosynthesis: photocatalytic production of hydrogen by plantinized photosystem I reaction centers. Photochem. Photobiol. 73, 630–635 (2001).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Noskov, V. N. et al. Defining the minimal length of sequence homology required for selective gene isolation by TAR cloning. Nucleic Acids Res. 29, 1–6 (2001).
Shanks, R. M. Q., Caiazza, N. C., Hinsa, S. M., Toutain, C. M. & O’Toole, G. A. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol. 72, 5027–5036 (2006).
Gietz, R. D. & Schiestl, R. H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007).
Williams, J. G. K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in synechocystis 6803. Methods Enzymol. 167, 766–778 (1988).
Gutekunst, K. et al. In-vivo turnover frequency of the cyanobacterial NiFe-hydrogenase during photohydrogen production outperforms in-vitro systems. Sci. Rep. 8, 1–10 (2018).
Eckert, C. et al. Genetic analysis of the Hox hydrogenase in the cyanobacterium synechocystis sp. PCC 6803 reveals subunit roles in association, assembly, maturation, and function. J. Biol. Chem. 287, 43502–43515 (2012).
Wittenberg, G. et al. Identification and characterization of a stable intermediate in photosystem I assembly in tobacco. Plant J. 90, 478–490 (2017).
Acknowledgements
We thank R. Schulz for giving us a scientific home. HoxH and HoxY antibodies were provided by P. Nixon. This study was financed by grants from the Bundesministerium für Bildung und Forschung (BMBF, FP3 09) and the Deutsche Forschungsgemeinschaft (GU1522/2-1).
Author information
Authors and Affiliations
Contributions
J.A. and K.G. conceived the research. J.A. and V.H. constructed and characterized the mutants. M.B. performed the immunoblot analysis. J.A., V.H., M.B. and K.G. analysed data. K.G. wrote the manuscript and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1–3.
Supplementary Data
Source data for Supplementary Fig. 5.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 8
Statistical Source Data
Rights and permissions
About this article
Cite this article
Appel, J., Hueren, V., Boehm, M. et al. Cyanobacterial in vivo solar hydrogen production using a photosystem I–hydrogenase (PsaD-HoxYH) fusion complex. Nat Energy 5, 458–467 (2020). https://doi.org/10.1038/s41560-020-0609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0609-6
This article is cited by
-
Prolonged hydrogen production by engineered green algae photovoltaic power stations
Nature Communications (2023)
-
Rewiring photosynthetic electron transport chains for solar energy conversion
Nature Reviews Bioengineering (2023)
-
Ascorbic and Salicylic Acids Vitalized Growth, Biochemical Responses, Antioxidant Enzymes, Photosynthetic Efficiency, and Ionic Regulation to Alleviate Salinity Stress in Sorghum bicolor
Journal of Plant Growth Regulation (2023)
-
GC/MS-based 13C metabolic flux analysis resolves the parallel and cyclic photomixotrophic metabolism of Synechocystis sp. PCC 6803 and selected deletion mutants including the Entner-Doudoroff and phosphoketolase pathways
Microbial Cell Factories (2022)
-
Three-dimensional ordered macroporous g-C3N4-Cu2O-TiO2 heterojunction for enhanced hydrogen production
Science China Materials (2022)