Abstract
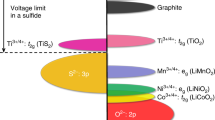
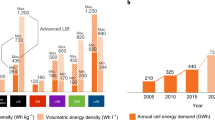
State-of-the-art lithium (Li)-ion batteries are approaching their specific energy limits yet are challenged by the ever-increasing demand of today’s energy storage and power applications, especially for electric vehicles. Li metal is considered an ultimate anode material for future high-energy rechargeable batteries when combined with existing or emerging high-capacity cathode materials. However, much current research focuses on the battery materials level, and there have been very few accounts of cell design principles. Here we discuss crucial conditions needed to achieve a specific energy higher than 350 Wh kg−1, up to 500 Wh kg−1, for rechargeable Li metal batteries using high-nickel-content lithium nickel manganese cobalt oxides as cathode materials. We also provide an analysis of key factors such as cathode loading, electrolyte amount and Li foil thickness that impact the cell-level cycle life. Furthermore, we identify several important strategies to reduce electrolyte-Li reaction, protect Li surfaces and stabilize anode architectures for long-cycling high-specific-energy cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whittingham, M. S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 114, 11414–11443 (2014).
Van Noorden, R. A better battery. Nature 507, 26–28 (2014).
Xia, C., Kwok, C. Y. & Nazar, L. F. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 361, 777–781 (2018).
Fang, R. et al. More reliable lithium-sulfur batteries: status, solutions and prospects. Adv. Mater. 29, 1606823 (2017).
Wu, B. et al. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A 4, 15266–15280 (2016).
Wu, B., Lochala, J., Taverne, T. & Xiao, J. The interplay between solid electrolyte interface (SEI) and dendritic lithium growth. Nano Energy 40, 34–41 (2017).
Wang, X. et al. New insights on the structure of electrochemically deposited lithium metal and its solid electrolyte interphases via cryogenic TEM. Nano Lett. 17, 7606–7612 (2017).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Yang, C. et al. Continuous plating/stripping behavior of solid-state lithium metal anode in a 3D ion-conductive framework. Proc. Natl Acad. Sci. USA 115, 3770–3775 (2018).
Kim, M. S. et al. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Pohl, A. et al. Development of a water based process for stable conversion cathodes on the basis of FeF3. J. Power Sources 313, 213–222 (2016).
Li, X. et al. Fundamental insight into Zr modification of Li- and Mn-rich cathodes: combined transmission electron microscopy and electrochemical impedance spectroscopy study. Chem. Mater. 30, 2566–2573 (2018).
Zhu, Z. et al. Anion-redox nanolithia cathodes for Li-ion batteries. Nat. Energy 1, 16111 (2016).
Yu, L. et al. A localized high-concentration electrolyte with optimized solvents and lithium difluoro(oxalate)borate additive for stable lithium metal batteries. ACS Energy Lett. 3, 2059–2067 (2018).
Nagpure, S. C. et al. Impacts of lean electrolyte on cycle life for rechargeable Li metal batteries. J. Power Sources 407, 53–62 (2018).
Elezgaray, J., Léger, C. & Argoul, F. Linear stability analysis of unsteady galvanostatic electrodeposition in the two-dimensional diffusion-limited regime. J. Electrochem. Soc. 145, 2016–2024 (1998).
Bai, P., Li, J., Brushett, F. R. & Bazant, M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016).
Burns, J. C. et al. Predicting and extending the lifetime of Li-ion batteries. J. Electrochem. Soc. 160, A1451–A1456 (2013).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Suo, L. et al. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl Acad. Sci. USA 115, 1156–1161 (2018).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Li, J., Li, W., You, Y. & Manthiram, A. Extending the service life of high-Ni layered oxides by tuning the electrode–electrolyte interphase. Adv. Energy Mater. 8, 1801957 (2018).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Liu, B., Zhang, J.-G. & Xu, W. Advancing lithium metal batteries. Joule 2, 833–845 (2018).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Qian, J. et al. Dendrite-free Li deposition using trace-amounts of water as an electrolyte additive. Nano Energy 15, 135–144 (2015).
Ren, X. et al. Guided lithium metal deposition and improved lithium Coulombic efficiency through synergistic effects of LiAsF6 and cyclic carbonate additives. ACS Energy Lett. 3, 14–19 (2018).
Liang, X. et al. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Ren, X. et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018).
Chen, S. et al. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2, 1548–1558 (2018).
Cheng, L. et al. Accelerating electrolyte discovery for energy storage with high-throughput screening. J. Phys. Chem. Lett. 6, 283–291 (2015).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Orsini, F. et al. In situ scanning electron microscopy (SEM) observation of interfaces within plastic lithium batteries. J. Power Sources 76, 19–29 (1998).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017).
Long, L., Wang, S., Xiao, M. & Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 4, 10038–10069 (2016).
Gomez, E. D. et al. Effect of ion distribution on conductivity of block copolymer electrolytes. Nano Lett. 9, 1212–1216 (2009).
Liu, K. et al. Lithium metal anodes with an adaptive “solid–liquid” interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017).
Wang, Y. & Sokolov, A. P. Design of superionic polymer electrolytes. Curr. Opin. Chem. Eng. 7, 113–119 (2015).
Aetukuri, N. B. et al. Flexible ion-conducting composite membranes for lithium batteries. Adv. Energy Mater. 5, 1500265 (2015).
Ye, H. et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 139, 5916–5922 (2017).
Liang, Z. et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl Acad. Sci. USA 113, 2862–2867 (2016).
Zhang, Y. et al. High-capacity, low-tortuosity, and channel-guided lithium metal anode. Proc. Natl Acad. Sci. USA 114, 3584–3589 (2017).
Zeng, Z. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018).
Acknowledgements
This research was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) under contract no. DE-AC02-05CH11231. The authors thank H. Pan, H. Lee, C. Niu and B. Liu of Pacific Northwest National Laboratory for their assistance in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Bao, Z., Cui, Y. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat Energy 4, 180–186 (2019). https://doi.org/10.1038/s41560-019-0338-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0338-x
This article is cited by
-
Cation replacement method enables high-performance electrolytes for multivalent metal batteries
Nature Energy (2024)
-
Hidden potential of lithium oxide
Nature Energy (2024)
-
Controlled large-area lithium deposition to reduce swelling of high-energy lithium metal pouch cells in liquid electrolytes
Nature Energy (2024)
-
Lithium inventory tracking as a non-destructive battery evaluation and monitoring method
Nature Energy (2024)
-
Metal electrodes for next-generation rechargeable batteries
Nature Reviews Electrical Engineering (2024)