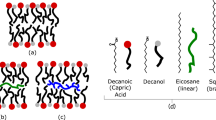
Abstract
Vesicles formed from single-chain amphiphiles (SCAs) such as fatty acids probably played an important role in the origin of life. A major criticism of the hypothesis that life arose in an early ocean hydrothermal environment is that hot temperatures, large pH gradients, high salinity and abundant divalent cations should preclude vesicle formation. However, these arguments are based on model vesicles using 1–3 SCAs, even though Fischer–Tropsch-type synthesis under hydrothermal conditions produces a wide array of fatty acids and 1-alkanols, including abundant C10–C15 compounds. Here, we show that mixtures of these C10–C15 SCAs form vesicles in aqueous solutions between pH ~6.5 and >12 at modern seawater concentrations of NaCl, Mg2+ and Ca2+. Adding C10 isoprenoids improves vesicle stability even further. Vesicles form most readily at temperatures of ~70 °C and require salinity and strongly alkaline conditions to self-assemble. Thus, alkaline hydrothermal conditions not only permit protocell formation at the origin of life but actively favour it.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available in the main text, Extended Data Figs. 1–10 and the Supplementary Information.
References
Mitchell, P. in The Origin of Life on the Earth (eds Oparin, A. I. et al.) 437–443 (Pergamon Press, 1957).
Nitschke, W. & Russell, M. J. Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge. J. Mol. Evol. 69, 481–496 (2009).
Fuchs, G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658 (2011).
Lane, N. & Martin, W. F. The origin of membrane bioenergetics. Cell 151, 1406–1416 (2012).
Mulkidjanian, A. Y., Galperin, M. Y. & Koonin, E. V. Co-evolution of primordial membranes and membrane proteins. Trends Biochem. Sci. 34, 206–215 (2009).
Koga, Y., Kyuragi, T., Nishihara, M. & Sone, N. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J. Mol. Evol. 46, 54–63 (1998).
Martin, W. & Russell, M. J. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. R. Soc. B 358, 59–85 (2003).
Koga, Y. Early evolution of membrane lipids: how did the lipid divide occur? J. Mol. Evol. 72, 274–282 (2011).
Sojo, V. On the biogenic origins of homochirality. Orig. Life Evol. Biosph. 45, 219–224 (2015).
Lombard, J., López-García, P. & Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 10, 507–515 (2012).
Shimada, H. & Yamagishi, A. Stability of heterochiral hybrid membrane made of bacterial sn-G3P lipids and archaeal sn-G1P lipids. ACS Biochem. 50, 4114–4120 (2011).
Caforio, A., Siliakus, M. F., Exterkate, M., Jain, S. & Jumde, V. R. Converting Escherichia coli into an archaebacterium with a hybrid heterochiral membrane. Proc. Natl Acad. Sci. USA 115, 3705–3709 (2018).
Hanczyc, M. M. & Szostak, J. W. Replicating vesicles as models of primitive cell growth and division. Curr. Opin. Chem. Biol. 8, 660–664 (2004).
Chen, I. A. & Szostak, J. W. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc. Natl Acad. Sci. USA 101, 7965–7970 (2004).
Chen, I. A. & Szostak, J. W. A kinetic study of the growth of fatty acid vesicles. Biophys. J. 87, 988–998 (2004).
Sojo, V., Pomiankowski, A. & Lane, N. A bioenergetic basis for membrane divergence in archaea and bacteria. PLoS Biol. 12, e1001926 (2014).
Segré, D., Ben-Eli, D., Deamer, D. W. & Lancet, D. The lipid world. Orig. Life Evol. Biosph. 31, 119–145 (2001).
Segré, D., Ben-Eli, D. & Lancet, D. Compositional genomes: prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl Acad. Sci. USA 97, 4112–4117 (2000).
Amend, J. P. & McCollom, T. M. Energetics of biomolecule synthesis on early earth. ACS Symp. Ser. 1025, 63–94 (2009).
Martin, W. Carbon–metal bonds: rare and primordial in metabolism. Trends Biochem. Sci. 44, 807–818 (2019).
McCollom, T. M., Ritter, G. & Simoneit, B. R. T. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch reactions. Orig. Life Evol. Biosph. 29, 153–166 (1999).
Scheidler, C., Sobotta, J., Eisenreich, W., Wächtershäuser, G. & Huber, C. Unsaturated C3,5,7,9-monocarboxylic acids by aqueous, one-pot carbon fixation: possible relevance for the origin of life. Sci. Rep. 6, 27595 (2016).
Decker, P. & Schweer, H. Reactions in the formol bioid: the origin of branched chains of isoprenoids, valine, and leucines. Orig. Life 14, 335–342 (1984).
Pray, H. A., Schweickert, C. E. & Minnich, B. H. Solubility of hydrogen, oxygen, nitrogen, and helium in water at elevated temperatures. Ind. Eng. Chem. 44, 1146–1151 (1952).
Baaske, P. et al. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl Acad. Sci. USA 104, 9346–9351 (2007).
Budin, I., Bruckner, R. J. & Szostak, J. W. Formation of protocell-like vesicles in a thermal diffusion column. J. Am. Chem. Soc. 131, 9628–9629 (2009).
Hanczyc, M. M., Mansy, S. S. & Szostak, J. W. Mineral surface directed membrane assembly. Orig. Life Evol. Biosph. 37, 67–82 (2007).
Buckel, W. & Thauer, R. K. Flavin-based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+ (Rnf) as electron acceptors: a historical review. Front. Microbiol. 9, 401 (2018).
West, T., Sojo, V., Pomiankowski, A. & Lane, N. The origin of heredity in protocells. Phil. Trans. R. Soc. B 372, 20160419 (2017).
Russell, M. J. & Hall, A. J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. London. 154, 377–402 (1997).
Russell, M. J. & Martin, W. The rocky roots of the acetyl-CoA pathway. Trends Biochem. Sci. 29, 358–363 (2004).
Russell, M. J., Daniel, R. M., Hall, A. J. & Sherringham, J. A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life. J. Mol. Evol. 39, 231–243 (1994).
Martin, W. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. Lond. B 362, 1887–1925 (2007).
Martin, W., Baross, J., Kelley, D. & Russell, M. J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814 (2008).
Russell, M. J. et al. The drive to life on wet and icy worlds. Astrobiology 14, 308–343 (2014).
Monnard, P.-A., Apel, C. L., Kanavarioti, A. & Deamer, D. W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2, 139–152 (2002).
Monnard, P. A. & Deamer, D. W. Membrane self-assembly processes: steps toward the first cellular life. Anat. Rec. 207, 123–151 (2002).
Szostak, J. W., Bartel, D. P. & Luisi, P. L. Synthesizing life. Nature 409, 387–390 (2001).
Deamer, D. The role of lipid membranes in life’s origin. Life 7, 5 (2017).
Milshteyn, D., Damer, B., Havig, J. & Deamer, D. Amphiphilic compounds assemble into membranous vesicles in hydrothermal hot spring water but not in seawater. Life 8, 11 (2018).
Maurer, S. The impact of salts on single chain amphiphile membranes and implications for the location of the origin of life. Life 7, 44 (2017).
Maurer, S. E. et al. Vesicle self-assembly of monoalkyl amphiphiles under the effects of high ionic strength, extreme pH, and high temperature environments. Langmuir 34, 15560–15568 (2018).
Apel, C. L., Deamer, D. W. & Mautner, M. N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1519, 1–9 (2002).
Rendón, A. et al. Model systems of precursor cellular membranes: long-chain alcohols stabilize spontaneously formed oleic acid vesicles. Biophys. J. 102, 278–286 (2012).
Namani, T. & Deamer, D. W. Stability of model membranes in extreme environments. Orig. Life Evol. Biosph. 38, 329–341 (2008).
Cape, J. L., Monnard, P. A. & Boncella, J. M. Prebiotically relevant mixed fatty acid vesicles support anionic solute encapsulation and photochemically catalyzed trans-membrane charge transport. Chem. Sci. 2, 661–671 (2011).
Monnard, P. A. & Deamer, D. W. Preparation of vesicles from nonphospholipid amphiphiles. Methods Enzymol. 372, 133–151 (2003).
Thanh Thuy, D., Decnop-Weever, D., Th. Kok, W., Luan, P. & Vong Nghi, T. Determination of traces of calcium and magnesium in rare earth oxides by flow-injection analysis. Anal. Chim. Acta 295, 151–157 (1994).
Avnir, Y. & Barenholz, Y. pH determination by pyranine: medium-related artifacts and their correction. Anal. Biochem. 347, 34–41 (2005).
Kelley, D. S. et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 412, 145–149 (2001).
Kelley, D. S. et al. A serpentinite-hosted ecosystem: the Lost City hydrothermal field. Science 307, 1428–1434 (2005).
Smith, R. & Tanford, C. Hydrophobicity of long chain n-alkyl carboxylic acids, as measured by their distribution between heptane and aqueous solutions. Proc. Natl Acad. Sci. USA 70, 289–293 (1973).
Budin, I., Prywes, N., Zhang, N. & Szostak, J. W. Chain-length heterogeneity allows for the assembly of fatty acid vesicles in dilute solution. Biophys. J. 107, 1582–1590 (2014).
Maurer, S. E. & Nguyen, G. Prebiotic vesicle formation and the necessity of salts. Orig. Life Evol. Biosph. 46, 215–222 (2016).
Hargreaves, W. R. & Deamer, D. W. Liposomes from ionic, single-chain amphiphiles. Biochemistry 17, 3759–3768 (1978).
Sojo, V., Herschy, B., Whicher, A., Camprubí, E. & Lane, N. The origin of life in alkaline hydrothermal vents. Astrobiology 16, 181–197 (2016).
Marty, B., Avice, G., Bekaert, D. V. & Broadley, M. W. Salinity of the Archaean oceans from analysis of fluid inclusions in quartz. C. R. Geosci. 350, 154–163 (2018).
Holland, H. D. The Chemical Evolution of the Atmosphere and Oceans (Princeton Univ. Press, 1984).
Fyfe, W. S. The water inventory of the Earth: fluids and tectonics. Geol. Soc. Spec. Publ. 78, 1–7 (1994).
Arndt, N. T. & Nisbet, E. G. Processes on the young earth and the habitats of early life. Annu. Rev. Earth Planet. Sci. 40, 521–549 (2012).
Russell, M. J. & Arndt, N. T. Geodynamic and metabolic cycles in the Hadean. Biogeosciences 2, 97–111 (2005).
Lopresti, C., Lomas, H., Massignani, M., Smart, T. & Battaglia, G. Polymersomes: nature inspired nanometer sized compartments. J. Mater. Chem. 19, 3576–3590 (2009).
Smart, T. et al. Block copolymer nanostructures. Nanotoday 3, 38–46 (2008).
Bonaccio, S., Wessicken, M., Berti, D., Walde, P. & Luisi, P. L. Relation between the molecular structure of phosphatidyl nucleosides and the morphology of their supramolecular and mesoscopic aggregates. Langmuir 12, 4976–4978 (1996).
Morse, J. W. & Mackenzie, F. T. Hadean ocean carbonate geochemistry. Aquat. Geochem. 4, 301–319 (1998).
Holm, N. G. The significance of Mg in prebiotic geochemistry. Geobiology 10, 269–279 (2012).
Ludwig, K. A., Kelley, D. S., Butterfield, D. A., Nelson, B. K. & Fru-Green, G. Formation and evolution of carbonate chimneys at the Lost City Hydrothermal Field. Geochim. Cosmochim. Acta 70, 3625–3645 (2006).
Adamala, K. & Szostak, J. W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 342, 1098–1101 (2013).
Jordan, S. F., Nee, E. & Lane, N. Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide. Interface Focus 9, 88–100 (2019).
Camprubi, E., Jordan, S. F., Vasiliadou, R. & Lane, N. Iron catalysis at the origin of life. IUBMB Life 69, 373–381 (2017).
Lane, N., Allen, J. F. & Martin, W. How did LUCA make a living? Chemiosmosis in the origin of life. BioEssays 32, 271–280 (2010).
Harrison, S. & Lane, N. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 9, 5176 (2018).
Acknowledgements
We thank M. Turmaine for assistance with NS-TEM, and B. Battaglia, F. Werner and D. Braben for discussions. We are grateful to the BBSRC (H.R., LIDo Doctoral Training Programme) and bgc3 for funding. A.M.H. and A.M. are funded by the Medical Research Council UK (Career Development Award grant no. MR/M00936X/1 to A.M.).
Author information
Authors and Affiliations
Contributions
N.L. supervised the work. S.F.J., H.R., I.N.Z., A.M.H., A.M. and N.L. conceived and designed the experiments. S.F.J., H.R., I.N.Z. and A.M.H. performed the experiments. S.F.J., I.N.Z., A.M.H. and A.M. contributed materials and analysis tools. S.F.J. and N.L. analysed the data. S.F.J. and N.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic representation of three different states of amphiphiles in aqueous solutions and their general relationship to pH.
Blue shading highlights hydrophilic areas whereas pink shading represents hydrophobic areas. Fully deprotonated amphiphiles will form micelles with a hydrophobic interior and hydrophilic exterior. Mixtures of protonated and deprotonated amphiphiles can self-organise into bilayer membranes forming vesicles with an aqueous interior. Fully protonated amphiphiles form hydrophobic droplets in aqueous solutions.
Extended Data Fig. 2 Optical density plot for 10:1 fatty acid/1-alkanol mixture.
Plot of normalised absorbance at 480 nm versus pH for 5 mM 10:1 C10-C15 fatty acid/1-alkanol mixture.
Extended Data Fig. 3 NS-TEM micrograph of vesicles from 0.1 mM 1:1 C10-C15 fatty acid/1-alkanol mixture.
NS-TEM micrograph of 0.1 mM 1:1 C10-C15 fatty acid/1-alkanol mixture vesicles at pH 7.17 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 4 NS-TEM micrograph of vesicles from 0.1 mM 1:1 C10-C15 fatty acid/1-alkanol mixture.
NS-TEM micrograph of 0.1 mM 1:1 C10-C15 fatty acid/1-alkanol mixture vesicles at pH 8.29 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 5 Critical bilayer concentration (CBC) plots for 1:1 and 5:1 fatty acid/1-alkanol mixtures.
Plot of absorbance at 480 nm versus concentration for 1:1 C10-C15 fatty acid/1-alkanol mixture (a) and 5:1 C10-C15 fatty acid/1-alkanol mixture (b).
Extended Data Fig. 6 NS-TEM micrograph of vesicles from 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture in NaCl.
NS-TEM micrograph of 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture vesicles in 600 mM NaCl at pH 12.19 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 7 NS-TEM micrograph of vesicles from 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture in MgCl2.
NS-TEM micrograph of 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture vesicles in 50 mM MgCl2 at pH 11.83 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 8 NS-TEM micrograph of vesicles from 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture in CaCl2.
NS-TEM micrograph of 5 mM 1:1 C10-C15 fatty acid/1-alkanol mixture vesicles in 10 mM CaCl2 at pH 12.18 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 9 NS-TEM micrograph of vesicles from 5 mM 1:1:1 C10-C15 fatty acid/1-alkanol/C10 isoprenoid mixture vesicles in mixture of salts.
NS-TEM micrograph of 5 mM 1:1:1 C10-C15 fatty acid/1-alkanol/C10 isoprenoid mixture vesicles in mixture of salts (600 mM NaCl, 50 mM MgCl2, 10 mM CaCl2) at pH 11.73 (some examples of vesicles indicated by black arrows).
Extended Data Fig. 10 Size exclusion chromatography (SEC) plot for 1:1:1 C10-C15 fatty acid/1-alkanol/C10 isoprenoid mixture vesicles in mixture of salts.
Plot of fluorescence intensity versus fraction number following size exclusion chromatography of 5 mM 1:1:1 C10-C15 fatty acid/1-alkanol/C10 isoprenoid mixture vesicles in mixture of salts (600 mM NaCl, 50 mM MgCl2, 10 mM CaCl2) at pH 12 prepared in the presence of 5 mM calcein. The initial peak represents the vesicle fraction and is followed by the free calcein dye.
Supplementary information
Supplementary Information
Supplementary methods, Tables 1 and 2 and Figs. 1–14.
Rights and permissions
About this article
Cite this article
Jordan, S.F., Rammu, H., Zheludev, I.N. et al. Promotion of protocell self-assembly from mixed amphiphiles at the origin of life. Nat Ecol Evol 3, 1705–1714 (2019). https://doi.org/10.1038/s41559-019-1015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-1015-y
This article is cited by
-
Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents
Communications Earth & Environment (2024)
-
Identification of a deep-branching thermophilic clade sheds light on early bacterial evolution
Nature Communications (2023)
-
Phosphorus availability on the early Earth and the impacts of life
Nature Geoscience (2023)
-
Formation of vesicular structures from fatty acids formed under simulated volcanic hydrothermal conditions
Scientific Reports (2023)
-
A fundamental limit to the search for the oldest fossils
Nature Ecology & Evolution (2022)