Abstract
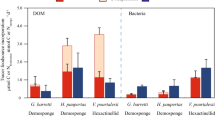
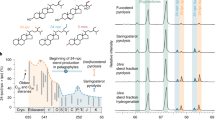
The dawn of animals remains one of the most mysterious milestones in the evolution of life. The fossil lipids 24-isopropylcholestane and 26-methylstigmastane are considered diagnostic for demosponges—arguably the oldest group of living animals. The widespread occurrence and high relative abundance of these biomarkers in Ediacaran sediments from 635–541 million years (Myr) ago have been viewed as evidence for the rise of animals to ecological importance approximately 100 Myr before their rapid Cambrian radiation. Here we show that the biosynthesis of 24-isopropylcholestane and 26-methylstigmastane precursors is common among early-branching unicellular Rhizaria—heterotrophic protists that play an important role in trophic cycling and carbon export in the modern ocean. Negating these hydrocarbons as sponge biomarkers, our study places the oldest evidence for animals closer to the Cambrian Explosion. Cambrian silica hexactine spicules that are approximately 535 Myr old now represent the oldest diagnostic sponge remains, whereas approximately 558-Myr-old Dickinsonia and Kimberella (Ediacara biota) provide the most reliable evidence for the emergence of animals. The proliferation of predatory protists may have been responsible for much of the ecological changes during the late Neoproterozoic, including the rise of algae, the establishment of complex trophic relationships and the oxygenation of shallow-water habitats required for the subsequent ascent of macroscopic animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data required to assess the interpretations made in this paper are included in the Supplementary Information. Additional (raw) data are available from the corresponding authors upon reasonable request.
References
Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014).
Butterfield, N. J. The Neoproterozoic. Curr. Biol. 25, R859–R863 (2015).
Antcliffe, J. B., Callow, R. H. & Brasier, M. D. Giving the early fossil record of sponges a squeeze. Biol. Rev. 89, 972–1004 (2014).
Botting, J. P. & Muir, L. A. Early sponge evolution: a review and phylogenetic framework. Palaeoworld 27, 1–29 (2017).
Erwin, D. H. et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
Peterson, K. J. et al. Estimating metazoan divergence times with a molecular clock. Proc. Natl Acad. Sci. USA 101, 6536–6541 (2004).
dos Reis, M. et al. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr. Biol. 25, 2939–2950 (2015).
Hedges, S. B., Blair, J. E., Venturi, M. L. & Shoe, J. L. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4, 2 (2004).
Love, G. D. et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721 (2009).
Zumberge, J. A. et al. Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nat. Ecol. Evol. 2, 1709–1714 (2018).
Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Grosjean, E., Love, G., Stalvies, C., Fike, D. & Summons, R. Origin of petroleum in the Neoproterozoic–Cambrian South Oman salt basin. Org. Geochem. 40, 87–110 (2009).
Gold, D. A. et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc. Natl Acad. Sci. USA 113, 2684–2689 (2016).
Grabenstatter, J. et al. Identification of 24-n-propylidenecholesterol in a member of the Foraminifera. Org. Geochem. 63, 145–151 (2013).
Sierra, R. et al. Deep relationships of Rhizaria revealed by phylogenomics: a farewell to Haeckel’s Radiolaria. Mol. Phylogenet. Evol. 67, 53–59 (2013).
Guidi, L. et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 532, 465–470 (2016).
Porter, S. M., Meisterfeld, R. & Knoll, A. H. Vase-shaped microfossils from the Neoproterozoic Chuar Group, Grand Canyon: a classification guided by modern testate amoebae. J. Paleontol. 77, 409–429 (2003).
Bosak, T. et al. Possible early foraminiferans in post-Sturtian (716−635 Ma) cap carbonates. Geology 40, 67–70 (2012).
Pawlowski, J. et al. The evolution of early Foraminifera. Proc. Natl Acad. Sci. USA 100, 11494–11498 (2003).
Groussin, M., Pawlowski, J. & Yang, Z. Bayesian relaxed clock estimation of divergence times in foraminifera. Mol. Phylogenet. Evol. 61, 157–166 (2011).
Caron, D. A. The rise of Rhizaria. Nature 532, 444–445 (2016).
Lampitt, R. S., Salter, I. & Johns, D. Radiolaria: major exporters of organic carbon to the deep ocean. Glob. Biogeochem. Cycles 23, GB1010 (2009).
Bobrovskiy, I. et al. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 361, 1246–1249 (2018).
Sperling, E., Robinson, J., Pisani, D. & Peterson, K. Where’s the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200‐Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8, 24–36 (2010).
Gold, D. A., O’Reilly, S. S., Luo, G., Briggs, D. E. G. & Summons, R. E. Prospects for sterane preservation in sponge fossils from museum collections and the utility of sponge biomarkers for molecular clocks. Bull. Peabody Mus. Nat. Hist. 57, 181–189 (2016).
Katz, M. E., Fennel, K. & Falkowski, P. G. in Evolution of Primary Producers in the Sea 405–430 (Elsevier, Amsterdam, 2007).
Hoshino, Y. et al. Cryogenian evolution of stigmasteroid biosynthesis. Sci. Adv. 3, e1700887 (2017).
Kodner, R. B., Pearson, A., Summons, R. E. & Knoll, A. H. Sterols in red and green algae: quantification, phylogeny, and relevance for the interpretation of geologic steranes. Geobiology 6, 411–420 (2008).
Volkman, J. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 60, 495–506 (2003).
Bobrovskiy, I., Hope, J. M., Krasnova, A., Ivantsov, A. & Brocks, J. J. Molecular fossils from organically preserved Ediacara biota reveal cyanobacterial origin for Beltanelliformis. Nat. Ecol. Evol. 2, 437–440 (2018).
Brocks, J. J. et al. Early sponges and toxic protists: possible sources of cryostane, an age diagnostic biomarker antedating Sturtian Snowball Earth. Geobiology 14, 129–149 (2016).
Lenton, T. M. & Daines, S. J. The effects of marine eukaryote evolution on phosphorus, carbon and oxygen cycling across the Proterozoic–Phanerozoic transition. Emerg. Top. Life. Sci. 2, 267–278 (2018).
Jürgens, K. & Massana, R. in Microbial Ecology of the Oceans 2nd edn (ed. Kirchman, D.) 383–442 (Wiley–Blackwell, Hoboken, NJ, USA, 2008).
Boraas, M. E., Seale, D. B. & Boxhorn, J. E. Phagotrophy by a flagellate selects for colonial prey: a possible origin of multicellularity. Evol. Ecol. 12, 153–164 (1998).
Grazhdankin, D. Patterns of distribution in the Ediacaran biotas: facies versus biogeography and evolution. Paleobiology 30, 203–221 (2004).
Cunningham, J. A., Liu, A. G., Bengtson, S. & Donoghue, P. C. The origin of animals: can molecular clocks and the fossil record be reconciled? BioEssays 39, 1–12 (2017).
Chang, S., Feng, Q., Clausen, S. & Zhang, L. Sponge spicules from the lower Cambrian in the Yanjiahe Formation, South China: the earliest biomineralizing sponge record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 474, 36–44 (2017).
Botting, J. P., Cárdenas, P. & Peel, J. S. A crown-group demosponge from the early Cambrian Sirius Passet biota, North Greenland. Palaeontology 58, 35–43 (2015).
Peters, K. E., Walters, C. C. & Moldowan, J. M. The Biomarker Guide. Volume 2: Biomarkers and Isotopes in Petroleum Exploration and Earth History (Cambridge Univ. Press, New York, 2005).
Hallmann, C., Kelly, A. E., Gupta, S. N. & Summons, R. E. in Quantifying the Evolution of Early Life 355–401 (Springer, Dordrecht, the Netherlands, 2011).
French, K. L. et al. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc. Natl Acad. Sci. USA 112, 5915–5920 (2015).
Acknowledgements
We thank P. Pringle and R. Tarozo for laboratory support; A. Leider, Y. Hoshino, M. Neumann, N. Kuznetsov and L. van Maldegem for discussions and reference samples; M. Holzmann, R. Sierra, J. Bernhard, S. Eggins, C. Bachy and C. Reymond for assistance in sourcing specimens; and S. Porter, R. Meisterfeld, S. Pruss, S. Chang and J. Botting for fossil images. This study was principally funded by the Max Planck Society (to C.H. and R.S.) and the Agouron Institute (Geobiology fellowship to B.J.N.). We also acknowledge the US National Science Foundation (grant nos. PLR134161 to S.S.B. and DBI-1349350 to M.W.L.), Swiss National Science Foundation (grant no. 31003A_179125 to J.P.), the German Research Foundation (grant no. NO1090/1-1 to E.N.), the Leibniz Association (grant no. SAW-2014-ISAS-2 to M.S.), Formas, the Swedish Research Council (A.S.), the French National Research Agency (grant no. IMPEKAB ANR-15-CE02-001 to F.N.) and Australian Research Council (grant nos. DP1095247 and DP160100607 to J.J.B.).
Author information
Authors and Affiliations
Contributions
B.J.N., C.H. and J.J.B. designed the study. A.S., F.N., M.L., C.S., R.S., E.C.M.N., P.D.D., J.P., S.S.B., K.Z. and M.S. cultured, collected and provided specimens. J.M.H. analysed Acantharea and I.B. analysed fossil algae. B.J.N. collected some specimens and analysed all other samples. B.J.N. and C.H. analysed and interpreted data. B.J.N., C.H. and J.J.B. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–8, Supplementary Tables 1 and 2, Supplementary Methods and Supplementary Text
Rights and permissions
About this article
Cite this article
Nettersheim, B.J., Brocks, J.J., Schwelm, A. et al. Putative sponge biomarkers in unicellular Rhizaria question an early rise of animals. Nat Ecol Evol 3, 577–581 (2019). https://doi.org/10.1038/s41559-019-0806-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-0806-5
This article is cited by
-
Common origin of sterol biosynthesis points to a feeding strategy shift in Neoproterozoic animals
Nature Communications (2023)
-
Sterol methyltransferases in uncultured bacteria complicate eukaryotic biomarker interpretations
Nature Communications (2023)
-
Current understanding on the Cambrian Explosion: questions and answers
PalZ (2021)
-
Algal origin of sponge sterane biomarkers negates the oldest evidence for animals in the rock record
Nature Ecology & Evolution (2020)
-
Geological alteration of Precambrian steroids mimics early animal signatures
Nature Ecology & Evolution (2020)