Abstract
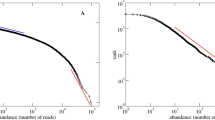
Marine plankton populate 70% of Earth’s surface, providing the energy that fuels ocean food webs and contributing to global biogeochemical cycles. Plankton communities are extremely diverse and geographically variable, and are overwhelmingly composed of low-abundance species. The role of this rare biosphere and its ecological underpinnings are however still unclear. Here, we analyse the extensive dataset generated by the Tara Oceans expedition for marine microbial eukaryotes (protists) and use an adaptive algorithm to explore how metabarcoding-based abundance distributions vary across plankton communities in the global ocean. We show that the decay in abundance of non-dominant operational taxonomic units, which comprise over 99% of local richness, is commonly governed by a power-law. Despite the high spatial turnover in species composition, the power-law exponent varies by less than 10% across locations and shows no biogeographical signature, but is weakly modulated by cell size. Such striking regularity suggests that the assembly of plankton communities in the dynamic and highly variable ocean environment is governed by large-scale ubiquitous processes. Understanding their origin and impact on plankton ecology will be important for evaluating the resilience of marine biodiversity in a changing ocean.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sunagawa, S. et al. Structure and function of the global ocean microbiome. Science 348, 1261359 (2015).
De Vargas, C. et al. Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1261605 (2015).
Fuhrman, J. A. Microbial community structure and its functional implications. Nature 459, 193–199 (2009).
Hutchinson, G. E. The paradox of the plankton. Am. Nat. 95, 137–145 (1961).
d’Ovidio, F., Monte, S. D., Alvain, S., Dandonneau, Y. & Lévy, M. Fluid dynamical niches of phytoplankton types. Proc. Natl Acad. Sci. USA 107, 18366–18370 (2010).
Hanson, C. A., Fuhrman, J. A., Horner-Devine, M. C. & Martiny, J. B. H. Beyond biogeographic patterns: processes shaping the microbial landscape. Nat. Rev. Microbiol. 10, 497–506 (2012).
McGillicuddy, D. J. Mechanisms of physical–biological–biogeochemical interaction at the oceanic mesoscale. Annu. Rev. Mar. Sci. 8, 125–159 (2016).
Galand, P. E., Casamayor, E. O., Kirchman, D. L. & Lovejoy, C. Ecology of the rare microbial biosphere of the Arctic Ocean. Proc. Natl Acad. Sci. USA 106, 22427–22432 (2009).
Pedrós-Alió, C. The rare bacterial biosphere. Annu. Rev. Mar. Sci. 4, 449–466 (2012).
Lennon, J. T. & Jones, S. E. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 9, 119–130 (2011).
Lynch, M. D. J. & Neufeld, J. D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 13, 217–229 (2015).
Sogin, M. L. et al. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl Acad. Sci. USA 103, 12115–12120 (2006).
Logares, R. et al. Patterns of rare and abundant marine microbial eukaryotes. Curr. Biol. 24, 813–821 (2014).
Barberán, A., Casamayor, E. O. & Fierer, N.The microbial contribution to macroecology. Front. Microbiol. 5, 203 (2014).
Chust, G., Irigoien, X., Chave, J. & Harris, R. P. Latitudinal phytoplankton distribution and the neutral theory of biodiversity. Glob. Ecol. Biogeogr. 22, 531–543 (2013).
Locey, K. J. & Lennon, J. T. Scaling laws predict global microbial diversity. Proc. Natl Acad. Sci. USA 113, 5970–5975 (2016).
Magurran, A. E. Measuring Biological Diversity (Blackwell, Oxford, 2004).
McGill, B. J. et al. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015 (2007).
Magurran, A. E. & McGill, B. J. Biological Diversity: Frontiers in Measurement and Assessment (Oxford Univ. Press, Oxford, 2011).
Volkov, I., Banavar, J. R., Hubbell, S. P. & Maritan, A. Neutral theory and relative species abundance in ecology. Nature 424, 1035–1037 (2003).
Pueyo, S. Diversity: between neutrality and structure. Oikos 112, 392–405 (2006).
Connolly, S. R. et al. Commonness and rarity in the marine biosphere. Proc. Natl Acad. Sci. USA 111, 8524–8529 (2014).
Ulrich, W., Ollik, M. & Ugland, K. I. A meta-analysis of species-abundance distributions. Oikos 119, 1149–1155 (2009).
Shoemaker, W. R., Locey, K. J. & Lennon, J. T. A macroecological theory of microbial biodiversity. Nat. Ecol. Evol. 1, 0107 (2017).
Matthews, T. J. & Whittaker, R. J. Neutral theory and the species abundance distribution: recent developments and prospects for unifying niche and neutral perspectives. Ecol. Evol. 4, 2263–2277 (2014).
Baldridge, E. et al. An extensive comparison of species-abundance distribution models. PeerJ 4, e2823 (2016).
Xiao, X., O’Dwyer, J. P. & White, E. P. Comparing process‐based and constraint‐based approaches for modeling macroecological patterns. Ecology 97, 1228–1238 (2016).
Azaele, S., Pigolotti, S., Banavar, J. R. & Maritan, A. Dynamical evolution of ecosystems. Nature 444, 926–928 (2006).
Magurran, A. E. & Henderson, P. A. Explaining the excess of rare species in natural species abundance distributions. Nature 422, 714–716 (2003).
Ulrich, W. & Ollik, M. Frequent and occasional species and the shape of relative‐abundance distributions. Divers. Distrib. 10, 263–269 (2004).
Mahé, F., Rognes, T., Quince, C., De Vargas, C. & Dunthorn, M. Swarmv2: highly-scalable and high-resolution amplicon clustering. PeerJ 3, e1420 (2015).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Woodward, G. et al. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 (2005).
White, E. P., Ernest, S. K. M., Kerkhoff, A. J. & Enquist, B. J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22, 323–330 (2007).
Matthews, T. J., Borges, P. A., Azevedo, E. B. & Whittaker, R. J. A biogeographical perspective on species abundance distributions: recent advances and opportunities for future research. J. Biogeogr. 44, 1705–1710 (2017).
Clauset, A., Shalizi, C. & Newman, M. Power-law distributions in empirical data. SIAM Rev. 51, 661–703 (2009).
Jeraldo, P. et al. Quantification of the relative roles of niche and neutral processes in structuring gastrointestinal microbiomes. Proc. Natl Acad. Sci. USA 109, 9692–9698 (2012).
He, F. Deriving a neutral model of species abundance from fundamental mechanisms of population dynamics. Funct. Ecol. 19, 187–193 (2005).
Azaele, S. et al. Statistical mechanics of ecological systems: neutral theory and beyond. Rev. Mod. Phys. 88, 035003 (2016).
Volkov, I., Banavar, J. R., He, F., Hubbell, S. P. & Maritan, A. Density dependence explains tree species abundance and diversity in tropical forests. Nature 438, 658–661 (2005).
Chaffron, S. et al. Environmental Context of Selected Samples from the Tara Oceans Expedition (2009–2013) (PANGAEA, 2014); https://doi.org/10.1594/PANGAEA.840718
Tittensor, D. P. et al. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 (2010).
Gravel, D., Canham, C. D., Beaudet, M. & Messier, C. Reconciling niche and neutrality: the continuum hypothesis. Ecol. Lett. 9, 399–409 (2006).
Wilkins, D., Van Sebille, E., Rintoul, S. R., Lauro, F. M. & Cavicchioli, R. Advection shapes Southern Ocean microbial assemblages independent of distance and environment effects. Nat. Commun. 4, 2457 (2013).
Ferriere, R. & Cazelles, B. Universal power laws govern intermittent rarity in communities of interacting species. Ecology 80, 1505–1521 (1999).
Lehahn, Y., d’Ovidio, F. & Koren, I.A satellite-based Lagrangian view on phytoplankton dynamics. Annu. Rev. Mar. Sci. 10, 99–119 (2018).
Cram, J. A. et al. Seasonal and interannual variability of the marine bacterioplankton community throughout the water column over ten years. ISME J. 9, 563–580 (2015).
Martin-Platero, A. M. et al. High resolution time series reveals cohesive but short-lived communities in coastal plankton. Nat. Commun. 9, 266 (2018).
Amaral-Zettler, L. A., McCliment, E. A., Ducklow, H. W. & Huse, S. M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 4, e6372 (2009).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Guillou, L. et al. The Protist Ribosomal Reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 41, D597–D604 (2013).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
McKane, A., Alonso, D. & Solé, R. V. Mean-field stochastic theory for species-rich assembled communities. Phys. Rev. E 62, 8466–8484 (2000).
Dennis, B. & Patil, G. P. The gamma distribution and weighted multimodal gamma distributions as models of population abundance. Math. Biosci. 68, 187–212 (1984).
Pueyo, S., He, F. & Zillio, T. The maximum entropy formalism and the idiosyncratic theory of biodiversity. Ecol. Lett. 10, 1017–1028 (2007).
Green, J. L. & Plotkin, J. B. A statistical theory for sampling species abundances. Ecol. Lett. 10, 1037–1045 (2007).
Goldstein, M. L., Morris, S. A. & Yen, G. G. Problems with fitting to the power-law distribution. Eur. Phys. J. B 41, 255–258 (2004).
Bauke, H. Parameter estimation for power-law distributions by maximum likelihood methods. Eur. Phys. J. B 58, 167–173 (2007).
Tsallis, C. & Stariolo, D. A. Generalized simulated annealing. Phys. A 233, 395–406 (1996).
Anderson, T. W. & Darling, D. A. Asymptotic theory of certain “goodness of fit” criteria based on stochastic processes. Ann. Math. Stat. 23, 193–212 (1952).
Acknowledgements
The authors are very grateful to F. d’Ovidio, V. Anjou and S. Audic, who participated in the early stages of this work, O. Missa, G. Sommeria-Klein and E. van Nimwegen for discussions on neutral models and model fitting, and the Tara Oceans consortium. The support of the informatics platform of IBENS has been essential for the computational part of this study. This work has received support under the programmes ‘Investissements d’Avenir’, launched by the French Government and implemented by ANR with the references ANR-10-LABX-54 MEMOLIFE, ANR-10-IDEX-0001-02 PSL* Research University and OCEANOMICS, as well as from the EU project MicroB3. C.B. additionally acknowledges funding from the ERC Advanced Award ‘Diatomite’, the Louis D Foundation of the Institut de France and the Radcliffe Institute of Advanced Study at Harvard University for a scholars fellowship during the 2016/17 academic year. This research was supported in part by National Science Foundation grant NSF PHY-1125915, NIH grant R25GM067110 and Gordon and Betty Moore Foundation grant 2919.01. This article is contribution number 76 of Tara Oceans.
Author information
Authors and Affiliations
Contributions
S.D.M. conceived and directed the study. E.S.-G. obtained the analytical results, designed the adaptive algorithm and produced the fits. S.M. carried out the preliminary analysis on the protist abundance distributions. L.Z. performed statistical analysis on the fitted parameters. S.D.M., E.S.-G., L.Z. and C.B. interpreted the results and wrote the manuscript. C.D.V. and E.K. provided access to the Tara Oceans dataset and commented on early versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods and Results, Supplementary Figures 1–4, Supplementary Tables 1–3, Supplementary References
Supplementary Data
This supplementary information provides all SADs, RADs and associated fits considered in this study
Rights and permissions
About this article
Cite this article
Ser-Giacomi, E., Zinger, L., Malviya, S. et al. Ubiquitous abundance distribution of non-dominant plankton across the global ocean. Nat Ecol Evol 2, 1243–1249 (2018). https://doi.org/10.1038/s41559-018-0587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0587-2
This article is cited by
-
Assembly processes and functional diversity of marine protists and their rare biosphere
Environmental Microbiome (2023)
-
A Lagrangian model for drifting ecosystems reveals heterogeneity-driven enhancement of marine plankton blooms
Nature Communications (2023)
-
Macroecological distributions of gene variants highlight the functional organization of soil microbial systems
The ISME Journal (2022)
-
Major restructuring of marine plankton assemblages under global warming
Nature Communications (2021)
-
Annual carbon retention of a marine-plankton community in the eutrophic Masan Bay, based on daily measurements
Marine Biology (2021)