Abstract
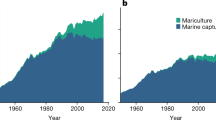
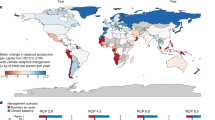
Marine aquaculture presents an opportunity for increasing seafood production in the face of growing demand for marine protein and limited scope for expanding wild fishery harvests. However, the global capacity for increased aquaculture production from the ocean and the relative productivity potential across countries are unknown. Here, we map the biological production potential for marine aquaculture across the globe using an innovative approach that draws from physiology, allometry and growth theory. Even after applying substantial constraints based on existing ocean uses and limitations, we find vast areas in nearly every coastal country that are suitable for aquaculture. The development potential far exceeds the space required to meet foreseeable seafood demand; indeed, the current total landings of all wild-capture fisheries could be produced using less than 0.015% of the global ocean area. This analysis demonstrates that suitable space is unlikely to limit marine aquaculture development and highlights the role that other factors, such as economics and governance, play in shaping growth trajectories. We suggest that the vast amount of space suitable for marine aquaculture presents an opportunity for countries to develop aquaculture in a way that aligns with their economic, environmental and social objectives.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Population Prospects: The 2015 Revision, Key Findings and Advance Tables (United Nations Department of Economic and Social Affairs, 2015).
Tilman, D. & Clark, M. Global diets link environmental sustainability and human health. Nature 515, 518–522 (2014).
The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All (Food and Agriculture Organization, 2016).
Maxwell, S. L., Fuller, R. A., Brooks, T. M. & Watson, J. E. M. The ravages of guns, nets and bulldozers. Nature 536, 143–145 (2016).
Pelletier, N. & Tyedmers, P. Forecasting potential global environmental costs of livestock production 2000–2050. Proc. Natl Acad. Sci. USA 107, 18371–18374 (2010).
Lovatelli, A., Aguilar-Manjarrez, J. & Soto, D. Expanding Mariculture Farther Offshore: Technical, Environmental, Spatial and Governance Challenges Technical Workshop 73 (FAO Fisheries and Aquaculture Department, 2013).
Merino, G. et al. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate? Glob. Environ. Chang. 22, 795–806 (2012).
Hall, S. J., Delaporte, A., Phillips, M. J., Beveridge, M. & O’Keefe, M. Blue Frontiers: Managing the Environmental Costs of Aquaculture (The WorldFish Center, Penang, Malaysia, 2011).
Tacon, A. G. J. & Metian, M. Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 21, 22–38 (2016).
Campbell, B. & Pauly, D. Mariculture: a global analysis of production trends since 1950. Mar. Policy 39, 94–100 (2013).
Primavera, J. H. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 49, 531–545 (2006).
Goldburg, R. J., Elliott, M. S. & Naylor, R. L. Marine Aquaculture in the United States: Environmental Impacts and Policy Options (Pew Oceans Commission, Arlington, Virginia, 2001).
Holmer, M. Environmental issues of fish farming in offshore waters: perspectives, concerns and research needs. Aquac. Environ. Interact. 1, 57–70 (2010).
Froehlich, H. E., Smith, A., Gentry, R. R. & Halpern, B. S. Offshore aquaculture: I know it when I see it. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00154 (2017).
Troell, M. et al. Does aquaculture add resilience to the global food system? Proc. Natl Acad. Sci. USA 111, 13257–13263 (2014).
Godfray, H. C. J. et al. Food security: the challenge of feeding 9 billion people. Science 327, 812–818 (2010).
Kapetsky, J. M., Agular-Manjarrez, J. & Jenness, J. A Global Assessment of Offshore Mariculture Potential from a Spatial Perspective (Food and Agriculture Organization, Rome, Italy, 2013).
Jiang, W. & Gibbs, M. T. Predicting the carrying capacity of bivalve shellfish culture using a steady, linear food web model. Aquaculture 244, 171–185 (2005).
Ferreira, J. G. et al. Analysis of coastal and offshore aquaculture: application of the FARM model to multiple systems and shellfish species. Aquaculture 289, 32–41 (2009).
Froehlich, H. E., Gentry, R. R. & Halpern, B. S. Synthesis and comparative analysis of physiological tolerance and life-history growth traits of marine aquaculture species. Aquaculture 460, 75–82 (2016).
Pauly, D. & Munro, J. L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 2, 21 (1984).
Pauly, D., Moreau, J. & Prein, M. A comparison of overall growth performance of Tilapia in open waters and aquaculture. In Second Int. Symp. Tilapia Aquaculture 469–479 (ICLARM Conference Proceedings, 1988).
Mathews, C. P. & Samuel, M. Using the growth performance index Φ’ to choose species aquaculture: an example from Kuwait. Aquabyte 3, 2–4 (1990).
Alvarez-Lajonchère, L. & Ibarra-Castro, L. Relationships of maximum length, length at first sexual maturity, and growth performance index in nature with absolute growth rates of intensive cultivation of some tropical marine fish. J. World Aquac. Soc. 43, 607–620 (2012).
Duarte, C. M., Marba, N. & Holmer, M. Rapid domestication of marine species. Science 316, 382–383 (2007).
Froehlich, H. E., Gentry, R. R., Rust, M. B., Grimm, D. & Halpern, S. Public perceptions of aquaculture: evaluating spatiotemporal patterns of sentiment around the world. PLoS ONE 12, e0169281 (2017).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Edwards, P. Aquaculture environment interactions: past, present and likely future trends. Aquaculture 447, 2–14 (2015).
O’Leary, B. C. et al. Effective coverage targets for ocean protection. Conserv. Lett. 9, 398–404 (2016).
Halpern, B. S. et al. A global map of human impact on marine ecosystems. Science 319, 948–952 (2008).
Halpern, B. S. et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 6, 7615 (2015).
Sanchez-Jerez, P. et al. Aquaculture’s struggle for space: the need for coastal spatial planning and the potential benefits of allocated zones for aquaculture (AZAs) to avoid conflict and promote sustainability. Aquac. Environ. Interact. 8, 41–54 (2016).
Halpern, B. S. et al. An index to assess the health and benefits of the global ocean. Nature 488, 615–620 (2012).
FAO Global Aquaculture Production Statistics Database Updated to 2013: Summary Information (FAO, 2015).
Krause, G. et al. A revolution without people? Closing the people–policy gap in aquaculture development. Aquaculture 447, 44–55 (2015).
Knapp, G. & Rubino, M. C. The political economics of marine aquaculture in the United States. Rev. Fish. Sci. Aquac. 24, 213–229 (2016).
Klinger, D. & Naylor, R. L. Searching for solutions in aquaculture: charting a sustainable course. Annu. Rev. Environ. Resour. 37, 247–276 (2012).
Bell, J. D. et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Chang. 3, 591–599 (2013).
Cheung, W. W. L. et al. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Chang. Biol. 16, 24–35 (2010).
Nguyen, H., Hien, P., Nang, T. & Lebailly, P. Vietnam’s fisheries and aquaculture development’s policy: are exports performance targets sustainable? In ISSAAS 2016: Int. Congress General Meeting (2016).
Golden, C. et al. Fall in fish catch threatens human health. Nature 534, 317–320 (2016).
Belton, B., Bush, S. R. & Little, D. C. Are the farmed fish just for the wealthy? Nature 538, 171 (2016).
Béné, C. et al. Contribution of fisheries and aquaculture to food security and poverty reduction: assessing the current evidence. World Dev. 79, 177–196 (2016).
IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer L. A.) (IPCC, 2015).
Fairbanks, L. Moving mussels offshore? Perceptions of offshore aquaculture policy and expansion in New England. Ocean Coast. Manag. 130, 1–12 (2016).
Naylor, R. L. et al. Feeding aquaculture in an era of finite resources. Proc. Natl Acad. Sci. USA 106, 15103–15110 (2009).
Ramos, J. et al. Perceived impact of offshore aquaculture area on small-scale fisheries: a fuzzy logic model approach. Fish. Res. 170, 217–227 (2015).
R Core Team R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2016); https://www.r-project.org/.
Locarnini, R. A. et al. World Ocean Atlas 2009 Volume 1: Temperature (2010); ftp://ftp.nodc.noaa.gov/pub/WOA09/DOC/woa09_vol1_text.pdf.
Rubino, M. Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities (US Department of Commerce National Oceanic and Atmospheric Administration, 2008).
Harris, J. O., Maguire, G., Edwards, S. J. & Johns, D. R. Low dissolved oxygen reduces growth rate and oxygen consumption rate of juvenile greenlip abalone, Haliotis laevigata Donovan. Aquaculture 174, 265–278 (1999).
Diaz, R. J. Overview of hypoxia around the world. J. Environ. Qual. 30, 275–281 (2001).
Vaquer-Sunyer, R. & Duarte, C. M. Thresholds of hypoxia for marine biodiversity. Proc. Natl Acad. Sci. USA 105, 15452–15457 (2008).
Blanchette, C. A., Helmuth, B. & Gaines, S. D. Spatial patterns of growth in the mussel, Mytilus californianus, across a major oceanographic and biogeographic boundary at Point Conception, California, USA. J. Exp. Mar. Bio. Ecol. 340, 126–148 (2007).
Page, H. M. & Hubbard, D. M. Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform: relationships to water temperature and food availability. J. Exp. Mar. Bio. Ecol. 111, 159–179 (1987).
Saxby, S. A. in A Review of Food Availability, Sea Water Characteristics and Bivalve Growth Performance at Coastal Culture Sites in Temperate and Warm Temperate Regions of the World 132 (Department of Fisheries, Western Australia, 2002).
Inglis, G. J., Hayden, B. J. & Ross, A. H. An Overview of Factors Affecting the Carrying Capacity of Coastal Embayments for Mussel Culture (National Institute of Water & Atmospheric Research, 2000).
Langan, R. The role of marine aquaculture in meeting the future demand for animal protein. J. Foodserv. 19, 227–233 (2008).
Puniwai, N. et al. Development of a GIS-based tool for aquaculture siting. ISPRS Int. J. Geoinf. 3, 800–816 (2014).
Kaiser, M. J., Snyder, B. & Yu, Y. A review of the feasibility, costs, and benefits of platform-based open ocean aquaculture in the Gulf of Mexico. Ocean Coast. Manag. 54, 721–730 (2011).
IUCN & UNEP World Database on Protected Areas (2009); http://www.wdpa.org/.
Day, J. et al. Guidelines for Applying the IUCN Protected Area Management Categories to Marine Protected Areas (IUCN, 2012).
Wood, L. J., Fish, L., Laughren, J. & Pauly, D. Assessing progress towards global marine protection targets: shortfalls in information and action. Oryx 42, 340–351 (2008).
Keys, A. B. The weight–length relation in fishes. Proc. Natl Acad. Sci. USA 14, 922–925 (1928).
Froese, R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol. 22, 241–253 (2006).
Gaspar, M. B., Santos, M. N. & Vasconcelos, P. Weight–length relationships of 25 bivalve species (Mollusca: Bivalvia) from the Algarve coast (southern Portugal). J. Mar. Biol. Assoc. UK 81, 805–807 (2001).
Commission Regulation (EC) No 710/2009 of 5 August 2009 Amending Regulation (EC) No 889/2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 15–34 (European Union, 2009).
Sim-Smith, C. & Forsythe, A. Comparison of the International Regulations and Best Management Practices for Marine Finfish Farming (New Zealand Ministry for Primary Industries, 2013).
FAO FishStatJ—Software for Fishery Statistical Time Series v.2.11.4 (2014); http://www.fao.org/fishery/statistics/software/fishstatj/en.
Acknowledgements
This research was conducted by the Open-Ocean Aquaculture Expert Working Group supported by the Science for Nature and People Partnership—a partnership of The Nature Conservancy, the Wildlife Conservation Society and the National Center for Ecological Analysis and Synthesis (proposal SNP015). The conclusions drawn in this manuscript do not necessarily reflect those of the author-associated organizations or their agencies. S.D.G. and R.R.G. acknowledge support from the Waitt Foundation. The authors thank R. Naylor and M. Velings for comments on an early draft of the manuscript.
Author information
Authors and Affiliations
Contributions
B.S.H. and R.R.G. conceived the initial study. R.R.G., H.E.F. and B.S.H. developed the research and methodology with critical input and insight from D.G., P.K, M.P., M.R. and S.D.G. R.R.G. and H.E.F. collected and analysed the data. All authors interpreted the results and implications. R.R.G., H.E.F., B.S.H. and S.D.G. produced the figures. R.R.G. drafted the manuscript with significant input and revisions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–8 and Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
Gentry, R.R., Froehlich, H.E., Grimm, D. et al. Mapping the global potential for marine aquaculture. Nat Ecol Evol 1, 1317–1324 (2017). https://doi.org/10.1038/s41559-017-0257-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0257-9
This article is cited by
-
Genetic improvement in edible fish: status, constraints, and prospects on CRISPR-based genome engineering
3 Biotech (2024)
-
Spatiotemporal variation of China’s mariculture potential under climate change
Reviews in Fish Biology and Fisheries (2024)
-
Reducing global land-use pressures with seaweed farming
Nature Sustainability (2023)
-
Islamic jurisprudence on the use of animal-derived ingredients in aquaculture feed
Aquaculture International (2023)
-
Expanding ocean food production under climate change
Nature (2022)