Abstract
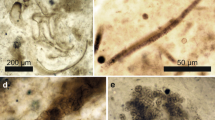
Gene sequences form the primary basis for understanding the relationships among extant plant groups, but genetic data are unavailable from fossils to evaluate the affinities of extinct taxa. Here we show that geothermally resistant fossil cuticles of seed-bearing plants, analysed with Fourier transform infrared (FTIR) spectroscopy and hierarchical cluster analysis (HCA), retain biomolecular suites that consistently distinguish major taxa even after experiencing different diagenetic histories. Our results reveal that similarities between the cuticular biochemical signatures of major plant groups (extant and fossil) are mostly consistent with recent phylogenetic hypotheses based on molecular and morphological data. Our novel chemotaxonomic data also support the hypothesis that the extinct Nilssoniales and Bennettitales are closely allied, but only distantly related to Cycadales. The chemical signature of the cuticle of Czekanowskia (Leptostrobales) is strongly similar to that of Ginkgo leaves and supports a close evolutionary relationship between these groups. Finally, our results also reveal that the extinct putative araucariacean, Allocladus, when analysed through HCA, is grouped closer to Ginkgoales than to conifers. Thus, in the absence of modern relatives yielding molecular information, FTIR spectroscopy provides valuable proxy biochemical data complementing morphological characters to distinguish fossil taxa and to help elucidate extinct plant relationships.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lindgren, J. et al. Molecular preservation of the pigment melanin in fossil melanosomes. Nat. Commun. 3, 824 (2012).
Lindgren, J. et al. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 506, 484–488 (2014).
van Bergen, P. F. et al. Resistant biomacromolecules in the fossil record. Acta Bot. Neerl. 44, 319–342 (1995).
Mösle, B., Finch, P., Collinson, M. E. & Scott, A. C. Comparison of modern and fossil plant cuticles by selective chemical extraction monitored by flash pyrolysis–gas chromatography–mass spectrometry and electron microscopy. J. Anal. Appl. Pyrol. 40–41, 585–597 (1997).
Mösle, B. et al. Factors influencing the preservation of plant cuticles: a comparison of morphology and chemical composition of modern and fossil examples. Org. Geochem. 29, 1369–1380 (1998).
Gupta, N. S., Collinson, M. E., Briggs, D. E. G., Evershed, R. P. & Pancost, R. D. Reinvestigation of the occurrence of cutan in plants: implications for the leaf fossil record. Paleobiol. 32, 432–449 (2006).
de Leeuw, J. W., Versteegh, G. J. M. & van Bergen, P. F. Biomacromolecules of algae and plants and their fossil analogues. Plant Ecol. 182, 209–233 (2006).
Amijaya, H., Schwarzbauer, J. & Littke, R. Organic geochemistry of the Lower Suban coal seam, South Sumatra Basin, Indonesia: palaeoecological and thermal metamorphism implications. Org. Geochem. 37, 261–279 (2006).
Lara, I., Belge, B. & Goulao, L. F. A focus on the biosynthesis and composition of cuticle in fruits. J. Agric. Food Chem. 63, 4005–4019 (2015).
Versteegh, G. J. M. & Riboulleau, A. An organic geochemical perspective on terrestrialization. Geol. Soc. Spec. Publ. 339, 11–36 (2010).
Crane, P. R. Phylogenetic analysis of seed plants and the origin of angiosperms. Ann. Mo. Bot. Gard. 72, 716–793 (1985).
Hilton, J. & Bateman, R. M. Pteridosperms are the backbone of seed-plant phylogeny. J. Torrey Bot. Soc. 133, 119–168 (2006).
Doyle, J. A. Seed ferns and the origin of angiosperms. J. Torrey Bot. Soc. 133, 169–209 (2006).
Briggs, D. E. G. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. R. Soc. Lond. B 354, 7–17 (1999).
Hatcher, P. G., Wilson, M. A., Vassallo, A. M. & Lerch, H. E. III. Studies of angiospermous wood in Australian brown coal by nuclear magnetic resonance and analytical pyrolysis: new insights into the early coalification process. Int. J. Coal Geol. 13, 99–126 (1989).
Qu, Y., Engdahl, A., Zhu, S., Vajda, V. & McLoughlin, N. Ultrastructural heterogeneity of carbonaceous material in ancient cherts: investigating biosignature origin and preservation. Astrobiology 15, 825–842 (2015).
Heredia-Guerrero, J. A. et al. Infrared and Raman spectroscopic features of plant cuticles: a review. Front. Plant Sci. 5, 305 (2014).
Alencar, W. J. et al. Spectroscopic analysis and X-ray diffraction of trunk fossils from the Parnaíba Basin, northeast Brazil. Spectrochim. Acta A Mol. Biomol. Spectrosc 135, 1052–1058 (2014).
Bomfleur, B., McLoughlin, S. & Vajda, V. Fossilized nuclei and chromosomes reveal 180 million years of genomic stasis in royal ferns. Science 343, 1376–1377 (2014).
Boyce, C. K. et al. Organic chemical differentiation within fossil plant cell walls detected with X-ray spectromicroscopy. Geology 30, 1039–1042 (2002).
D’Angelo, J. A., Zodrow, E. L. & Camargo, A. Chemometric study of functional groups in Pennsylvanian gymnosperm plant organs (Sydney Coalfield, Canada): implications for chemotaxonomy and assessment of kerogen formation. Org. Geochem. 41, 1312–1325 (2010).
D’Angelo, J. A. & Zodrow, E. L. Chemometric study of functional groups in different layers of Trigonocarpus grandis ovules (Pennsylvanian seed fern, Canada). Org. Geochem. 42, 1039–1054 (2011).
D’Angelo, J. A. & Zodrow, E. L. Chemometric study of structural groups in medullosalean foliage (Carboniferous, fossil Lagerstätte, Canada): chemotaxonomic implications. Int. J. Coal Geol. 138, 42–54 (2015).
Lomax, B. H. et al. A novel palaeoaltimetry proxy based on spore and pollen wall chemistry. Earth Planet. Sci. Lett. 353–354, 22–28 (2012).
Steemans, P., Lepot, K., Marshall, C. P., Le Hérissé, A. & Javaux, E. J. FTIR characterisation of the chemical composition of Silurian miospores (cryptospores and trilete spores) from Gotland, Sweden. Rev. Palaeobot. Palynol. 162, 577–590 (2010).
Seyfullah, L. J., Sadowski, E.-M. & Schmidt, A. Species-level determination of closely related araucarian resins using FTIR spectroscopy and its implications for the provenance of New Zealand amber. Peer J. 3, e1067 (2015).
Lu, H.-F., Shen, J.-B., Lin, X.-Y. & Fu, J.-L. Relevance of fourier transform infrared spectroscopy and leaf anatomy for species classification in Camellia (Theaceae). Taxon 57, 1274–1288 (2008).
Zimmermann, B. & Kohler, A. Infrared spectroscopy of pollen identifies plant species and genus as well as environmental conditions. PLoS ONE 9, e95417 (2014).
Niklas, K. J. & Gensel, P. G. Chemotaxonomy of some Paleozoic vascular plants. Part II: chemical characterization of major plant groups. Brittonia 29, 100–111 (1977).
Jones, W. G., Hill, K. D. & Allen, J. M. Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea (Syd.) 6, 173–176 (1995).
Escapa, I. H. & Catalano, S. A. Phylogenetic analysis of Araucariaceae: integrating molecules, morphology, and fossils. Int. J. Plant Sci. 174, 1153–1170 (2013).
Hermsen, E. J., Taylor, T. N., Taylor, E. L. & Stevenson, D. W. Cataphylls of the Middle Triassic cycad Antarcticycas schopfii and new insights into cycad evolution. Am. J. Bot. 93, 724–738 (2006).
Chaw, S.-M., Parkinson, C. L., Cheng, Y., Vincent, T. M. & Palmer, J. D. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc. Natl Acad. Sci. USA 97, 4086–4091 (2000).
Morris, J. Remarks upon the recent and fossil cycadaceae. Ann. Mag. Nat. Hist. 7, 110–120 (1841).
Delevoryas, T. Investigations of North American cycadeoids: cones of cycadeoidea. Am. J. Bot. 50, 45–52 (1963).
Kimura, T. & Sekido, S. Nilssoniocladus n. gen. (Nilssoniaceae, n. fam.), newly found from the early Lower Cretaceous of Japan. Palaeontographica B 153, 111–118 (1975).
Pott, C., McLoughlin, S., Lindström, A., Wu, S.-Q. & Friis, E. M. Baikalophyllum lobatum and Rehezamites anisolobus: two seed plants with “cycadophyte” foliage from the Early Cretaceous of Eastern Asia. Int. J. Plant Sci. 173, 192–208 (2012).
Kustatscher, E. & van Konijenburg-van Cittert, J. H. A. Taxonomical and palaeogeographic considerations on the seedfern genus Ptilozamites with some comments on Anomozamites, Dicroidium, Pseudoctenis and Ctenozamites. Neues Jahrb. Geol. Paläontol. Abh. 243, 71–100 (2007).
Rees, P. M., Ziegler, A. M. & Valdes, P. J. Warm Climates in Earth History (eds Huber, B. T., Macleod, K. G. & Wing, S. L.) (Cambridge Univ. Press, 2000).
Liu, X.-Q., Li, C.-S. & Wang, Y.-F. Plants of Leptostrobus Heer (Czekanowskiales) from the Early Cretaceous and Late Triassic of China, with discussion of the genus. J. Integr. Plant Biol. 48, 137–147 (2006).
Chaw, S. M., Zharkikh, A., Sung, H. M., Lau, T. C. & Li, W. H. Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18S rRNA sequences. Mol. Biol. Evol. 14, 56–68 (1997).
Ruhfel, B. R., Gitzendanner, M. A., Soltis, P. S., Soltis, D. E. & Burleigh, J. G. From algae to angiosperms—inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol. Biol. 14, 23 (2014).
Wu, C.-S., Chaw, S.-M. & Huang, Y.-Y. Chloroplast phylogenomics indicates that Ginkgo biloba is sister to cycads. Genome Biol. Evol. 5, 243–254 (2013).
Wickett, N. J. et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111, E4859–E4868 (2014).
Jansson, I.-M., McLoughlin, S., Vajda, V. & Pole, M. An Early Jurassic flora from the Clarence-Moreton Basin, Australia. Rev. Palaeobot. Palynol. 150, 5–21 (2008).
Pattemore, G. A., Rigby, J. F. & Playford, G. Palissya: a global review and reassessment of eastern Gondwanan material. Rev. Palaeobot. Palynol. 210, 50–61 (2014).
Baker, M. J. et al. Using fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 9, 1771–1791 (2014).
Madejová, J. & Komadel, P. Baseline studies of the clay minerals society source clays: infrared methods. Clays Clay Miner. 49, 410–432 (2001).
Domenighini, A. & Giordano, M. Fourier transform infrared spectroscopy of microalgae as a novel tool for biodiversity studies, species identification, and the assessment of water quality. J. Phycol. 45, 522–531 (2009).
Bar-Joseph, Z., Gifford, D. K. & Jaakkola, T. S. Fast optimal leaf ordering for hierarchical clustering. Bioinformatics 17, S22–S29 (2001).
Acknowledgements
This research was financially supported by grants from Lund University, faculty funding for synchrotron-light-related projects for ESS and MAX IV to V.V., the Swedish Research Council (VR grant 2015-4264 to V.V., and VR grant 2014-5234 to S.M.), This research was further funded by the Swedish Research Council (VR) under grant LUCCI (Lund University Carbon Cycle Centre) and the Utrecht Network Young researchers’ grant to M.P. We thank the Botanical Garden of Lund University, Sweden for giving permission to sample plant leaves; M. Pole (Nanjing Institute of Geology and Paleontology, Nanjing, China) for providing fossil cuticles from New Zealand and Svend Visby Funder (Centre for GeoGenetics, Natural History Museum, Copenhagen, Denmark) for giving access to T. M. Harris’ fossil collections.
Author information
Authors and Affiliations
Contributions
V.V. and P.U. conceived and led the project, V.V. and S.M. recovered and prepared fossil cuticles, V.V. collected the modern cuticles. M.P., V.V. and A.E. carried out FTIR spectroscopy measurements, A.E., P.U. and J.H. provided expertise in infrared spectroscopy. Spectral analysis was performed by M.P., P.U. and J.H.S. All authors contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
1 Supplementary Table, 4 Supplementary Figures, Supplementary References and Supplementary details of analysis
Rights and permissions
About this article
Cite this article
Vajda, V., Pucetaite, M., McLoughlin, S. et al. Molecular signatures of fossil leaves provide unexpected new evidence for extinct plant relationships. Nat Ecol Evol 1, 1093–1099 (2017). https://doi.org/10.1038/s41559-017-0224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0224-5
This article is cited by
-
Molecular fingerprints resolve affinities of Rhynie chert organic fossils
Nature Communications (2023)
-
Middle Jurassic palaeoclimate changes within the central Ordos Basin based on palynological records
Palaeobiodiversity and Palaeoenvironments (2023)
-
Cretaceous gnetalean yields first preserved plant gum
Scientific Reports (2020)
-
Ginkgo leaf cuticle chemistry across changing pCO2 regimes
PalZ (2019)
-
An introduction to Jurassic biodiversity and terrestrialenvironments
Palaeobiodiversity and Palaeoenvironments (2018)