Abstract
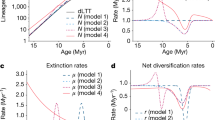
The foundations of several disciplines can be expressed as simple quantitative laws, for example, Newton's laws or the laws of thermodynamics. Here I present five laws derived from fossil data that describe the relationships among species extinction and longevity, species richness, origination rates, extinction rates and diversification. These statements of our palaeobiological knowledge constitute a dimension largely hidden from view when studying the living biota, which are nonetheless crucial to the study of evolution and ecology even for groups with poor or non-existent fossil records. These laws encapsulate: the critical fact of extinction; that species are typically geologically short-lived, and thus that the number of extinct species typically dwarfs the number of living species; that extinction and origination rates typically have similar magnitudes; and, that significant extinction makes it difficult to infer much about a clade's early history or its current diversity dynamics from the living biota alone. Although important strides are being made to integrate these core palaeontological findings into our analysis of the living biota, this knowledge needs to be incorporated more widely if we are to understand their evolutionary dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Slater, G. J., Harmon, L. J. & Alfaro, M. E. Integrating fossils with molecular phylogenies improves inferences of trait evolution. Dryadhttp://doi.org/10.5061/dryad.q96d7 (2012).
Stadler, T. Recovering speciation and extinction dynamics based on phylogenies. J. Evol. Biol. 26, 1203–1219 (2013).
Morlon, H. Phylogenetic approaches for studying diversification. Ecol. Lett. 17, 508–525 (2014).
Bacon, C. D. et al. Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc. Natl Acad. Sci. USA 112, 6110–6115 (2015).
Macleod, N. The geological extinction record: History, data, biases, and testing. Geol. Soc. Am. Spec. Pap. 505, SPE505-01 (2014).
Bailey, N. T. J. The Elements of Stochastic Processes, with Applications to the Natural Sciences (Wiley, 1964).
Raup, D. M. Mathematical models of cladogenesis. Paleobiology 11, 42–52 (1985). Pioneering paper in the statistical analysis of evolutionary birth–death processes.
Van Valen, L. A new evolutionary law. Evol. Theory 1, 1–30 (1973).
Raup, D. M. Taxonomic survivorship curves and Van Valen's Law. Paleobiology 1, 82–96 (1975).
Kendall, D. G. On the generalized ‘birth-and-death’ process. Ann. Math. Stat. 19, 1–15 (1948).
Alroy, J. Quantitative Mammalian Biochronology and Biogeography of North America (Univ. Chicago, 1994).
Alroy, J. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 285–311 (1996).
Bradley, R. et al. Revised checklist of North American mammals north of Mexico, 2014. Occas. Pap. Museum Texas Tech Univ. 327, 1–27 (2014).
Foote, M. & Miller, A. I. Principles of Paleontology (W. H. Freeman and Company, 2007). Comprehensive introduction to the analysis of the fossil record.
Meredith, R. W. et al. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524 (2011).
dos Reis, M. et al. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B 279, 3491–3500 (2012).
Buckley, L. B. et al. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138 (2010).
Jetz, W. & Fine, P. V. A. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 (2012).
Kammer, T. W., Baumiller, T. K. & Ausich, W. I. Evolutionary significance of differential species longevity in Osagean–Meramecian (Mississippian) crinoid clades. Paleobiology 24, 155–176 (1998).
Crampton, J. S., Cooper, R. A., Sadler, P. M. & Foote, M. Greenhouse-icehouse transition in the Late Ordovician marks a step change in extinction regime in the marine plankton. Proc. Natl Acad. Sci. USA 113, 1498–1503 (2016).
Horowitz, A. S., Blakely, R. F. & Macurda, D. B. J. Taxonomic survivorship within the Blastoidea (Echinodermata). J. Paleontol. 59, 543–550 (1985).
Norris, R. D. Biased extinction and evolutionary trends. Paleobiology 17, 388–399 (1991).
Silvestro, D., Cascales-Miñana, B., Bacon, C. D. & Antonelli, A. Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436 (2015).
Niklas, K. J., Tiffney, B. H. B. H. & Knoll, A. H. Patterns in vascular land plant diversification. Nature 303, 614–616 (1983).
Marshall, C. R. & Quental, T. B. The uncertain role of diversity dependence in species diversification and the need to incorporate time-varying carrying capacities. Philos. Trans. B 371,(2016).
Quental, T. B. & Marshall, C. R. Diversity dynamics: Molecular phylogenies need the fossil record. Trends Ecol. Evol. 25, 435–441 (2010). Summarizes the fact that many evolutionary processes can lead to similar looking phylogenies.
Rabosky, D. L. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 (2014).
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Morlon, H., Parsons, T. L. & Plotkin, J. B. Reconciling molecular phylogenies with the fossil record. Proc. Natl Acad. Sci. USA 108, 16327–32 (2011).
Reyes, E., Morlon, H. & Sauquet, H. Presence in Mediterranean hotspots and floral symmetry affect speciation and extinction rates in Proteaceae. New Phytol. 207, 401–410 (2015).
Liow, L. H., Quental, T. B. & Marshall, C. R. When can decreasing diversification rates be detected with molecular phylogenies and the fossil record? Syst. Biol. 59, 646–659 (2010).
Quental, T. B. & Marshall, C. R. How the Red Queen drives terrestrial mammals to extinction. Science 341, 290–292 (2013).
Lim, J. Y. & Marshall, C. R. The true tempo of evolutionary radiation and decline revealed on the Hawaiian Archipelago. Nature 543, 710–713 (2017).
Heath, T. A., Huelsenbeck, J. P. & Stadler, T. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl Acad. Sci. USA 111, E2957–E2966 (2014).
Zhang, C., Stadler, T., Klopfstein, S., Heath, T. A. & Ronquist, F. Total-evidence dating under the fossilized birth–death process. Syst. Biol. 65, 228–249 (2016).
Losos, J. B. Seeing the forest for the trees: the limitations of phylogenies in comparative biology. (American Society of Naturalists Address). Am. Nat. 177, 709–27 (2011).
Foote, M. Origination and extinction through the Phanerozoic: a new approach. J. Geol. 111, 125–148 (2003).
Foote, M. Pulsed origination and extinction in the marine realm. Paleobiology 31, 6–20 (2005).
Lu, P. J., Yogo, M. & Marshall, C. R. Phanerozoic marine biodiversity dynamics in light of the incompleteness of the fossil record. Proc. Natl Acad. Sci. USA 103, 2736–2739 (2006).
Condamine, F. L., Nagalingum, N. S., Marshall, C. R. & Morlon, H. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 15, 65 (2015).
Marshall, C. & Schultze, H.-P. Relative importance of molecular, neontological, and paleontological data in understanding the biology of the vertebrate invasion of land. J. Mol. Evol. 35, 93–101 (1992).
Coates, M. I. & Clack, J. A. Fish-like gills and breathing in the earliest known tetrapod. Nature 352, 234–236 (1991).
Coates, M. I. & Clack, J. A. Polydactyly in the earliest known tetrapod limbs. Nature 347, 66–69 (1990).
Campbell, K. S. W. & Barwick, R. E. Geological and palaeontological information and phylogenetic hypotheses. Geol. Mag. 125, 207–227 (1988).
Rozhnov, S. V. Symmetry of echinoderms: From initial bilaterally-asymmetric metamerism to pentaradiality. Nat. Sci. 6, 171–183 (2014).
Brusatte, S. L., O’Connor, J. K. & Jarvis, E. D. The origin and diversification of birds. Curr. Biol. 25, R888–R898 (2015).
White, T. D., Lovejoy, C. O., Asfaw, B., Carlson, J. P. & Suwa, G. Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proc. Natl Acad. Sci. USA 112, 4877–4884 (2015).
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
Steeman, M. E. et al. Radiation of extant cetaceans driven by restructuring of the oceans. Syst. Biol. 58, 573–585 (2009).
Quental, T. B. & Marshall, C. R. The molecular phylogenetic signature of clades in decline. PLoS ONE 6, e25780 (2011).
Bininda-emonds, O. R. P. et al. The delayed rise of present-day mammals. Nature 446, 507–512 (2007).
Alroy, J. The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Syst. Biol. 48, 107–118 (1999).
Halliday, T. J. D., Upchurch, P. & Goswami, A. Eutherians experienced elevated evolutionary rates in the immediate aftermath of the Cretaceous–Palaeogene mass extinction. Proc. R. Soc. B 283, 20153026 (2016).
Alroy, J. Accurate and precise estimates of origination and extinction rates. Paleobiology 40, 374–397 (2014).
Alroy, J. A more precise speciation and extinction rate estimator. Paleobiology 41, 633–639 (2015).
Silvestro, D., Schnitzler, J., Liow, L. H., Antonelli, A. & Salamin, N. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Syst. Biol. 63, 349–367 (2014).
Silvestro, D., Antonelli, A., Salamin, N. & Quental, T. B. The role of clade competition in the diversification of North American canids. Proc. Natl Acad. Sci. USA 112, 8684–8689 (2015).
Acknowledgements
This manuscript has benefited from feedback from S. Finnegan, S. Holland, J. Y. Lim, T. Quental, and especially from S.-P. Quek, D. Varajao de Latorre and reviews from M. Foote, D. Silvestro.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Marshall, C. Five palaeobiological laws needed to understand the evolution of the living biota. Nat Ecol Evol 1, 0165 (2017). https://doi.org/10.1038/s41559-017-0165
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0165
This article is cited by
-
Primitive purine biosynthesis connects ancient geochemistry to modern metabolism
Nature Ecology & Evolution (2024)
-
The importance of continents, oceans and plate tectonics for the evolution of complex life: implications for finding extraterrestrial civilizations
Scientific Reports (2024)
-
The multicausal twilight of South American native mammalian predators (Metatheria, Sparassodonta)
Scientific Reports (2022)
-
Phylogenetic relations and range history of jerboas of the Allactaginae subfamily (Dipodidae, Rodentia)
Scientific Reports (2022)
-
Evolvability and Macroevolution: Overview and Synthesis
Evolutionary Biology (2022)