Abstract
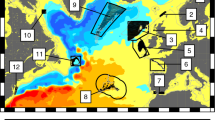
Larval dispersal is a critical yet enigmatic process in the persistence and productivity of marine metapopulations. Empirical data on larval dispersal remain scarce, hindering the use of spatial management tools in efforts to sustain ocean biodiversity and fisheries. Here we document dispersal among subpopulations of clownfish (Amphiprion percula) and butterflyfish (Chaetodon vagabundus) from eight sites across a large seascape (10,000 km2) in Papua New Guinea across 2 years. Dispersal of clownfish was consistent between years, with mean observed dispersal distances of 15 km and 10 km in 2009 and 2011, respectively. A Laplacian statistical distribution (the dispersal kernel) predicted a mean dispersal distance of 13–19 km, with 90% of settlement occurring within 31–43 km. Mean dispersal distances were considerably greater (43–64 km) for butterflyfish, with kernels declining only gradually from spawning locations. We demonstrate that dispersal can be measured on spatial scales sufficient to inform the design of and test the performance of marine reserve networks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Botsford, L. W. et al. Connectivity, sustainability, and yield: bridging the gap between conventional fisheries management and marine protected areas. Rev. Fish. Biol. Fish. 19, 69–95 (2009).
Kritzer, J. & Sale, P. F. Metapopulation ecology in the sea: from Levin’s model to marine ecology and fisheries science. Fish Fisheries 5, 131–140 (2004).
Gell, F. R. & Roberts, C. M. Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol. Evol. 18, 448–455 (2003).
Sale, P. F. et al. Critical science gaps impede use of no-take marine reserves. Trends Ecol. Evol. 20, 74–80 (2005).
Beger, M. et al. Incorporating asymmetric connectivity into spatial decision making for conservation. Cons. Lett. 3, 359–368 (2010).
Magris, R. A., Pressey, R. L., Weeks, R. & Ban, N. C. Integrating connectivity and climate change into marine conservation planning. Biol. Cons. 170, 207–221 (2014).
Roberts, C. M. Connectivity and management of Caribbean coral reefs. Science 278, 1454–1457 (1997).
Cowen, R. K. & Sponaugle, S. Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 1, 443–466 (2009).
Cowen, R. K., Paris, C. B. & Srinivasan, A. Scaling of connectivity in marine populations. Science 311, 522–527 (2006).
Levin, L. A. Recent progress in understanding larval dispersal: new directions and digressions. Integr. Comp. Biol. 46, 282–297 (2006).
Pinsky, M. L., Palumbi, S. R., Andrefouet, S. & Purkis, S. J. Open and closed seascapes: where does habitat patchiness create populations with high fractions of self-recruitment? Ecol. Applic. 22, 1257–1267 (2012).
Lett, C., Nguyen-Huu, T., Cuif, M., Saenz-Agudelo, P. & Kaplan, D. M. Linking local retention, self-recruitment, and persistence in marine metapopulations. Ecology 96, 2236–2244 (2015).
Burgess, S. C. et al. Beyond connectivity: how empirical methods can quantify population persistence to improve marine protected area design. Ecol. Appl. 24, 257–270 (2014).
Simpson, S. D., Harrison, H. B., Claereboudt, M. R. & Planes, S. Long-distance dispersal via ocean currents connects clownfish populations throughout entire species range. PLoS ONE 9, e107610 (2014).
Jones, G. P. et al. Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325 (2009).
Jones, G. P. in Ecology of Fishes on Coral Reefs: The Functioning of an Ecosystem in a Changing World (ed. Mora, C. ) 16–27 (Cambridge Univ. Press, 2015).
Jones, G. P., Milicich, M. J., Emslie, M. J. & Lunow, C. Self-recruitment in a coral reef fish population. Nature 402, 802–804 (1999).
Almany, G. R., Berumen, M. L., Thorrold, S. R., Planes, S. & Jones, G. P. Local replenishment of coral reef fish populations in a marine reserve. Science 316, 742–744 (2007).
Jones, G. P., Thorrold, S. R. & Planes, S. Coral reef fish larvae settle close to home. Curr. Biol. 15, 1314–1318 (2005).
Planes, S., Jones, G. P. & Thorrold, S. R. Larval dispersal connects fish populations in a network of marine protected areas. Proc. Natl Acad. Sci. USA 106, 5693–5697 (2009).
Christie, M. R. et al. Larval connectivity in an effective network of marine protected areas. PLoS ONE 5, e15715 (2010).
Berumen, M. L. et al. Persistence of self-recruitment and patterns of larval connectivity in a marine protected area network. Ecol. Evol. 2, 444–452 (2012).
Saenz Agudelo, P., Jones, G. P., Thorrold, S. R. & Planes, S. Connectivity dominates larval replenishment in a coastal coral reef fish metapopulation. Proc. R. Soc. B. 278, 2954–2961 (2010).
Harrison, H. B. et al. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023–1028 (2012).
D’Aloia, C. C. et al. Patterns, causes, and consequences of marine larval dispersal. Proc. Natl Acad. Sci. USA 112, 13940–19945 (2015).
Almany, G. R. et al. Dispersal of grouper larvae drives local resource sharing in a coral reef fishery. Curr. Biol. 23, 626–630 (2013).
Bonin, M. C. et al. The role of marine reserves in the replenishment of a locally impacted population of anemonefish on the Great Barrier Reef. Mol. Ecol. 25, 487–499 (2016).
Wabnitz, C ., Taylor, M ., Green, E & Razak, T. From Ocean to Aquarium: The Global Trade in Marine Ornamental Species (UNEP-WCMC, 2003).
Jones, G. P., McCormick, M. I., Srinivasan, M. & Eagle, J. V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl Acad. Sci. USA 101, 8251–8253 (2004).
Green, A. et al. Designing a resilient network of marine protected areas for Kimbe Bay, Papua New Guinea. Oryx 43, 488–498 (2009).
Gerber, S., Chabrier, P. & Kremer, A. Famoz: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Mol. Ecol. Notes 3, 479–481 (2003).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007).
Nanninga, G. B., Saenz-Agudelo, P., Zhan, P., Hoteit, I. & Berumen, M. L. Not finding Nemo: limited reef-scale retention in a coral reef fish. Coral Reefs 34, 383–392 (2015).
Salles, O. C. et al. Coral reef populations can persist without immigration. Proc. R. Soc. B. 282, 2015131 (2015).
Williamson, D. H. et al. Large-scale, multidirectional larval connectivity among coral reef fish populations in the Great Barrier Reef Marine Park. Mol. Ecol. 25, 6039–6054 (2016).
Pinsky, M. L. et al. Marine dispersal scales are congruent over evolutionary and ecological time. Curr. Biol. 27, 1–6 (2017).
Steinberg, C. R ., Choukroun, S. M ., Slivkoff, M. M ., Mahoney, M. V. & Brinkman, R. M. Currents in the Bismarck Sea and Kimbe Bay, Papua New Guinea. TNC Pacific Islands Countries Report 6/06 (Australian Institute of Marine Science/The Nature Conservancy, 2006).
Lobel, P. S. & Robinson, A. R. Transport and entrainment of fish larvae by ocean mesoscale eddies and currents. Deep-Sea Res. 33, 483–500 (1986).
Shulzitski, K., Sponaugle, S., Hauf, M., Walter, K. D. & Cowen, R. K. Encounter with mesoscale eddies enhances survival in larval coral reef fishes. Proc. Natl Acad. Sci. USA 113, 6928–6933 (2016).
Gerlach, G., Atema, J., Kingsford, M. J., Black, K. P. & Miller-Sims, V. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA 104, 858–863 (2007).
Dixson, D. L. et al. Coral reef fish smell leaves to find island homes. Proc. R. Soc. B 275, 2831–2839 (2008).
Berumen, M. L. et al. Otolith geochemistry does not reflect dispersal history of clownfish larvae. Coral Reefs 29, 883–891 (2010).
Lester, S. E. et al. Biological effects within no-take reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46 (2009).
Emslie, M. J. et al. Expectation and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Park. Curr. Biol. 25, 983–992 (2015).
Cowen, R. K., Gawarkiewicz, G., Pineda, J., Thorrold, S. R. & Werner, F. E. Population connectivity in marine systems: an overview. Oceanography 20, 14–21 (2009).
Kaplan, D. M., Botsford, L. W. & Jorgensen, S. Dispersal-per-recruit: an efficient method for assessing sustainability in networks of marine reserves. Ecol. Appl. 16, 2248–2263 (2006).
Almany, G. R. Marine ecology: reserves are necessary, but not sufficient. Curr. Biol. 25, R328–R347 (1998).
Harrison, H. B., Saenz-Agudelo, P., Planes, S., Jones, G. P. & Berumen, M. L. Relative accuracy of three common methods of parentage analysis in natural populations. Mol. Ecol. 22, 1158–1170 (2013).
McCormick, M. I. & Choat, J. H. Estimating total abundance of a large temperate reef fish using visual strip-transects. Mar. Biol. 96, 469–478 (1987).
Acknowledgements
We thank the volunteers who dedicated long hours in the water collecting tissue samples: R. Brooker, S. Choukroun, P. Costello, J. Davies, D. Dixson, K. Furby, M. Giru, B. Grover, J. Hill, N. Jones, K. McMahon, M. Noble, S. Noonan, N. Raventos Klein, M. Pinsky, J. Roberts, J. Smith, N. Tolou, M. Takahashi, P. Waldie and M. White; and the people of the villages on the shores of Kimbe Bay who welcomed us into their communities and supported this research: Kilu-Tamare, Lolobau, Tairobe and Vaiaku. This research would not have been possible without the support of the Walindi Plantation Resort, the skipper and crew of MV Febrina, Mahonia Na Dari Research and Conservation Centre, and The Nature Conservancy. This work was supported by Australian Research Council funding to G.P.J., the King Abdullah University of Science and Technology (baseline research funds to M.L.B. and a Special Partnership Collaborative Fellowship to M.L.B. and P.S.-A.) and NSF grants OCE0928442 and OCE1031256 to S.R.T.
Author information
Authors and Affiliations
Contributions
G.R.A., M.L.B., G.P.J., S.P. and S.R.T designed the study. All authors contributed to field work and editing of the manuscript. M.B., H.B.H., S.P., M.A.P., P.S.-A. and S.P. conducted microsatellite DNA analyses. G.R.A. and M.B. developed the dispersal kernel model. M.B., H.H. and S.R.T. created figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Tables 1–6; Supplementary Figures 1,2; Supplementary Methods (PDF 618 kb)
Rights and permissions
About this article
Cite this article
Almany, G., Planes, S., Thorrold, S. et al. Larval fish dispersal in a coral-reef seascape. Nat Ecol Evol 1, 0148 (2017). https://doi.org/10.1038/s41559-017-0148
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0148
This article is cited by
-
Tropical seamounts as stepping-stones for coral reef fishes: range extensions and new regional distributions from mesophotic ecosystems in the Coral Sea, Australia
Marine Biodiversity (2024)
-
Positive spatial autocorrelation in three habitat quality indicators sets the stage for evolution of adaptive dispersal plasticity in a coral reef fish
Coral Reefs (2024)
-
Local Overfishing Patterns Have Regional Effects on Health of Coral, and Economic Transitions Can Promote Its Recovery
Bulletin of Mathematical Biology (2022)
-
Rank change and growth within social hierarchies of the orange clownfish, Amphiprion percula
Marine Biology (2022)
-
An integrative investigation of sensory organ development and orientation behavior throughout the larval phase of a coral reef fish
Scientific Reports (2021)