Abstract
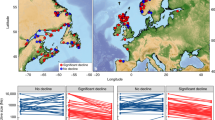
Interbreeding between domesticated and wild animals occurs in several species. This gene flow has long been anticipated to induce genetic changes in life-history traits of wild populations, thereby influencing population dynamics and viability. Here, we show that individuals with high levels of introgression (domesticated ancestry) have altered age and size at maturation in 62 wild Atlantic salmon Salmo salar populations, including seven ancestral populations to breeding lines of the domesticated salmon. This study documents widespread changes to life-history traits in wild animal populations following gene flow from selectively bred, domesticated conspecifics. The continued high abundance of escaped, domesticated Atlantic salmon thus threatens wild Atlantic salmon populations by inducing genetic changes in fitness-related traits. Our results represent key evidence and a timely warning concerning the potential ecological impacts of the globally increasing use of domesticated animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kidd, A. G., Bowman, J., Lesbarreres, D. & Schulte-Hostedde, A. I. Hybridization between escaped domestic and wild American mink (Neovison vison). Mol. Ecol. 18, 1175–1186 (2009).
Verardi, A., Lucchini, V. & Randi, E. Detecting introgressive hybridization between free-ranging domestic dogs and wild wolves (Canis lupus) by admixture linkage disequilibrium analysis. Mol. Ecol. 15, 2845–2855 (2006).
Canu, A. et al. Are captive wild boar more introgressed than free-ranging wild boar? Two case studies in Italy. Eur. J. Wildlife. Res. 60, 459–467 (2014).
Lecis, R. et al. Bayesian analyses of admixture in wild and domestic cats (Felis silvestris) using linked microsatellite loci. Mol. Ecol. 15, 119–131 (2006).
Halbert, N. D. & Derr, J. N. A comprehensive evaluation of cattle introgression into US federal bison herds. J. Hered. 98, 1–12 (2007).
Glover, K. A. et al. Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genet. 14, 74 (2013).
Karlsson, S., Diserud, O. H., Fiske, P. & Hindar, K. Widespread genetic introgression of escaped farmed Atlantic salmon in wild salmon populations. ICES J. Mar. Sci. 73, 2488–2498 (2016).
Taberlet, P. et al. Are cattle, sheep, and goats endangered species? Mol. Ecol. 17, 275–284 (2008).
Tufto, J. Effects of releasing maladapted individuals: a demographic-evolutionary model. Am. Nat. 158, 331–340 (2001).
Tufto, J. Gene flow from domesticated species to wild relatives: migration load in a model of multivariate selection. Evolution 64, 180–192 (2010).
Ellstrand, N. C. Dangerous Liaisons?: When Cultivated Plants Mate with their Wild Relatives (Johns Hopkins Univ. Press, 2003).
Gjøen, H. M. & Bentsen, H. B. Past, present, and future of genetic improvement in salmon aquaculture. ICES J. Mar. Sci. 54, 1009–1014 (1997).
Gjedrem, T. The first family-based breeding program in aquaculture. Rev. Aquacult. 2, 2–15 (2010).
Moen, T., Baranski, M., Sonesson, A. K. & Kjoglum, S. Confirmation and fine-mapping of a major QTL for resistance to infectious pancreatic necrosis in Atlantic salmon (Salmo salar): population-level associations between markers and trait. BMC Genom. 10, 368 (2009).
Thodesen, J., Grisdale-Helland, B., Helland, S. J. & Gjerde, B. Feed intake, growth and feed utilization of offspring from wild and selected Atlantic salmon (Salmo salar). Aquaculture 180, 237–246 (1999).
Solberg, M. F., Skaala, O., Nilsen, F. & Glover, K. A. Does domestication cause changes in growth reaction norms? A study of farmed, wild and hybrid Atlantic salmon families exposed to environmental stress. PLoS ONE 8, e54469 (2013).
Einum, S. & Fleming, I. A. Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. J. Fish Biol. 50, 634–651 (1997).
Fraser, D. J., Minto, C., Calvert, A. M., Eddington, J. D. & Hutchings, J. A. Potential for domesticated-wild interbreeding to induce maladaptive phenology across multiple populations of wild Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 67, 1768–1775 (2010).
Yates, M. C., Debes, P. V., Fraser, D. J. & Hutchings, J. A. The influence of hybridization with domesticated conspecifics on alternative reproductive phenotypes in male Atlantic salmon in multiple temperature regimes. Can. J. Fish. Aquat. Sci. 72, 1–8 (2015).
Debes, P. V. & Hutchings, J. A. Effects of domestication on parr maturity, growth, and vulnerability to predation in Atlantic salmon. Can. J. Fish. Aquat. Sci. 71, 1371–1384 (2014).
McGinnity, P. et al. Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES J. Mar. Sci. 54, 998–1008 (1997).
McGinnity, P. et al. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. R. Soc. B 270, 2443–2450 (2003).
Fleming, I. A. et al. Lifetime success and interactions of farm salmon invading a native population. Proc. R. Soc. B 267, 1517–1523 (2000).
Skaala, O. et al. Performance of farmed, hybrid, and wild Atlantic salmon (Salmo salar) families in a natural river environment. Can. J. Fish. Aquat. Sci. 69, 1994–2006 (2012).
Legendre, S. in Evolutionary Conservation Biology (eds Ferrière, R., Dieckmann, U. & Couvet, D. ) 41–58 (Cambridge Univ. Press, 2005).
Jonsson, N., Hansen, L. P. & Jonsson, B. Variation in age, size and repeat spawning of adult Atlantic salmon in relation to river discharge. J. Anim. Ecol. 60, 937–947 (1991).
Hutchings, J. A. & Jones, M. E. B. Life history variation and growth rate thresholds for maturity in Atlantic salmon, Salmo salar. Can. J. Fish. Aquat. Sci. 55 (suppl.), 22–47 (1998).
Fleming, I. A., Jonsson, B., Gross, M. R. & Lamberg, A. An experimental study of the reproductive behaviour and success of farmed and wild Atlantic salmon (Salmo salar). J. Appl. Ecol. 33, 893–905 (1996).
Jonsson, B., Jonsson, N. & Albretsen, J. Environmental change influences the life history of salmon Salmo salar in the North Atlantic Ocean. J. Fish Biol. 88, 618–637 (2016).
Beamish, R. J., Mahnken, C. & Neville, C. M. Evidence that reduced early marine growth is associated with lower marine survival of coho salmon. Trans. Am. Fish. Soc. 133, 26–33 (2004).
Hansen, L. P., Jonsson, B., Morgan, R. I. G. & Thorpe, J. E. Influence of parr maturity on emigration of smolting Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 46, 410–415 (1989).
Clifford, S. L., McGinnity, P. & Ferguson, A. Genetic changes in Atlantic salmon (Salmo salar) populations of northwest Irish rivers resulting from escapes of adult farm salmon. Can. J. Fish. Aquat. Sci. 55, 358–363 (1998).
Crozier, W. W. Escaped farmed salmon, Salmo salar L., in the Glenarm River, Northern Ireland: genetic status of the wild population 7 years on. Fish. Manage. Ecol. 7, 437–446 (2000).
Skaala, O., Wennevik, V. & Glover, K. A. Evidence of temporal genetic change in wild Atlantic salmon, Salmo salar L., populations affected by farm escapees. ICES J. Mar. Sci. 63, 1224–1233 (2006).
Bourret, V., O’Reilly, P. T., Carr, J. W., Berg, P. R. & Bernatchez, L. Temporal change in genetic integrity suggests loss of local adaptation in a wild Atlantic salmon (Salmo salar) population following introgression by farmed escapees. Heredity 106, 500–510 (2011).
Glover, K. A. et al. Three decades of farmed escapees in the wild: a spatio-temporal analysis of Atlantic salmon population genetic structure throughout Norway. PLoS ONE 7, e43129 (2012).
Report of the Working Group on North Atlantic Salmon (WGNAS) ICES CM 2016/ACOM:10 (ICES 2016).
Skilbrei, O. T., Heino, M. & Svasand, T. Using simulated escape events to assess the annual numbers and destinies of escaped farmed Atlantic salmon of different life stages from farm sites in Norway. ICES J. Mar. Sci. 72, 670–685 (2015).
Gjedrem, T., Gjøen, H. M. & Gjerde, B. Genetic origin of Norwegian farmed Atlantic salmon. Aquaculture 98, 41–50 (1991).
Bourret, V. et al. SNP-array reveals genome-wide patterns of geographical and potential adaptive divergence across the natural range of Atlantic salmon (Salmo salar). Mol. Ecol. 22, 532–551 (2013).
Baskett, M. L., Burgess, S. C. & Waples, R. S. Assessing strategies to minimize unintended fitness consequences of aquaculture on wild populations. Evol. Appl. 6, 1090–1108 (2013).
Baskett, M. L. & Waples, R. S. Evaluating alternative strategies for minimizing unintended fitness consequences of cultured individuals on wild populations. Conserv. Biol. 27, 83–94 (2013).
Huisman, J. & Tufto, J. Comparison of non-Gaussian quantitative genetic models for migration and stabilizing selection. Evolution 66, 3444–3461 (2012).
Karlsson, S., Diserud, O. H., Moen, T. & Hindar, K. A standardized method for quantifying unidirectional genetic introgression. Ecol. Evol. 4, 3256–3263 (2014).
Fraser, D. J. et al. Consequences of farmed-wild hybridization across divergent wild populations and multiple traits in salmon. Ecol. Appl. 20, 935–953 (2010).
Barson, N. J. et al. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 528, 405–408 (2015).
Schindler, D. E. et al. Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612 (2010).
The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All (FAO, 2016).
Naylor, R. et al. Fugitive salmon: assessing the risks of escaped fish from net-pen aquaculture. Bioscience 55, 427–437 (2005).
Jensen, A. J. (ed.) Geographical Variation and Population Trends in Norwegian Atlantic Salmon (NINA, 2004).
Karlsson, S., Moen, T., Lien, S., Glover, K. A. & Hindar, K. Generic genetic differences between farmed and wild Atlantic salmon identified from a 7K SNP-chip. Mol. Ecol. Resour. 11, 247–253 (2011).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Nielsen, E. E., Bach, L. A. & Kotlicki, P. HYBRIDLAB (version 1.0): a program for generating simulated hybrids from population samples. Mol. Ecol. Notes 6, 971–973 (2006).
Report of the Workshop on Age Determination of Salmon (WKADS) ICES CM 2011/ACOM:44 (ICES, 2011).
Lund, R. A. & Hansen, L. P. Identification of wild and reared Atlantic salmon, Salmo salar L., using scale characters. Aquacult. Res. 22, 499–508 (1991).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2003).
Kristensen, K., Nielsen, A., Berg, C. W., Skaug, H. & Bell, B. M. TMB: automatic differentiation and laplace approximation. J. Stat. Softw. 70, JSSv070i05 (2016).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016).
Bates, D., Machler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, JSSv076i01 (2015).
Acknowledgements
We thank G. M. Østborg and J. G. Jensås for scale reading, T. Balstad, L. B. Eriksen and M. H. Spets for genetic analyses and J. D. Linnell for discussion. The study was financed by the Research Council of Norway (grant 216105, QuantEscape), the Norwegian Environment Agency and the Norwegian Institute for Nature Research.
Author information
Authors and Affiliations
Contributions
G.H.B., K.H., O.H.D. and S.K. conceived the study. S.K. and O.H.D. generated and conducted bioinformatics on the molecular data. K.H., H.S., P.F., A.J.J., K.U., T.F.N., B.T.B., B.F.-L., H.L. and E.N. coordinated the collection of phenotypic data. G.H.B. analysed the data. G.H.B., K.H., G.R., B.J. and S.K. wrote the manuscript. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1,2; Supplementary Tables 1–4; Supplementary Methods; Supplementary References (PDF 484 kb)
Rights and permissions
About this article
Cite this article
Bolstad, G., Hindar, K., Robertsen, G. et al. Gene flow from domesticated escapes alters the life history of wild Atlantic salmon. Nat Ecol Evol 1, 0124 (2017). https://doi.org/10.1038/s41559-017-0124
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0124
This article is cited by
-
Characterization of pure and admixed brown trout (Salmo trutta) populations of high conservation value in the upper Danubian contact zone using ddRADseq genotyping
Hydrobiologia (2024)
-
The meaning of wild: Genetic and adaptive consequences from large-scale releases of domestic mallards
Communications Biology (2023)
-
First releases of hatchery-produced Senegal sole (Solea senegalensis), brill (Scophthalmus rhombus), and wedge sole (Dicologoglossa cuneata) juveniles in the South-western Spanish coast
Hydrobiologia (2023)
-
Landscape and stocking effects on population genetics of Tennessee Brook Trout
Conservation Genetics (2022)
-
A review of marine stressors impacting Atlantic salmon Salmo salar, with an assessment of the major threats to English stocks
Reviews in Fish Biology and Fisheries (2022)