Abstract
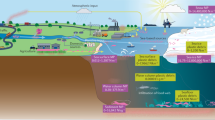
Marine microscopic plastic (microplastic) debris is a modern societal issue, illustrating the challenge of balancing the convenience of plastic in daily life with the prospect of causing ecological harm by careless disposal. Here we develop the concept of microplastic as a complex, dynamic mixture of polymers and additives, to which organic material and contaminants can successively bind to form an ‘ecocorona’, increasing the density and surface charge of particles and changing their bioavailability and toxicity. Chronic exposure to microplastic is rarely lethal, but can adversely affect individual animals, reducing feeding and depleting energy stores, with knock-on effects for fecundity and growth. We explore the extent to which ecological processes could be impacted, including altered behaviours, bioturbation and impacts on carbon flux to the deep ocean. We discuss how microplastic compares with other anthropogenic pollutants in terms of ecological risk, and consider the role of science and society in tackling this global issue in the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carpenter, E. J. & Smith, K. L. Plastics on the Sargasso Sea surface. Science 175, 1240–1241 (1972).
Plastics — The Facts 2014/2015 (PlasticsEurope, 2015).
Waters, C. N. et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, aad2622 (2016).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). Combines data on waste production with a model that uses population density and economic status to estimate the amount of land-based plastic waste entering the ocean.
Eriksen, M. et al. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9, e111913 (2014).
Thompson, R. C. et al. Lost at sea: where is all the plastic?. Science 304, 838 (2004).
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Andrady, A. L. in Marine Anthropogenic Litter (eds Bergmann, M., Gutow, L. & Klages, M. ) 57–72 (Springer, 2015).
Gewert, B., Plassmann, M. M. & MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Processes Impacts 17, 1513–1521 (2015).
Harshvardhan, K. & Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 77, 100–106 (2013).
Wright, S. L., Thompson, R. C. & Galloway, T. S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 178, 483–492 (2013).
Lusher, A. in Marine Anthropogenic Litter (eds Bergmann, M., Gutow, L. & Klages, M. ) 245–307 (Springer, 2015).
Lynch, I. et al. in Nanoscience and the Environment (eds Lead, J. R. & Valsami-Jones, E. ) Ch. 4, 127–156 (Frontiers of Nanoscience Vol. 7, 2014). Introduces the ecocorona as an important concept that helps to explain how nanoparticles behave in the environment.
Zettler, E. R., Mincer, T. J. & Amaral-Zettler, L. A. Life in the ‘plastisphere’: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146 (2013).
Koelmans, A. A., Bakir, A., Burton, G. A. & Janssen, C. R. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 50, 3315–3326 (2016).
Galloway, T. S. in Marine Anthropogenic Litter (eds Bergmann, M., Gutow, L. & Klages, M. ) 343–366 (Springer, 2015).
Rochman, C. M. et al. Policy: classify plastic waste as hazardous. Nature 494, 169–171 (2013).
Rochman, C. M. et al. The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived. Ecology 97, 302–312 (2016).
Cózar, A. et al. Plastic debris in the open ocean. Proc. Natl Acad. Sci. USA 111, 10239–10244 (2014). Provides a first-order approximation of how much plastic pollution there is in surface waters resulting in a global map of high-density areas.
Gigault, J., Pedrono, B., Maxit, B. & Ter Halle, A. Marine plastic litter: the unanalyzed nano-fraction. Environ. Sci. Nano 3, 346–350 (2016).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Koelmans, A. A., Besseling, E. & Shim, W. J. in Marine Anthropogene Litter (eds Bergmann, M., Gutow, L. & Klages, M. ) 325–340 (Springer, 2015).
Wegner, A., Besseling, E., Foekema, E., Kamermans, P. & Koelmans, A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 31, 2490–2497 (2012).
Turner, J. T. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Prog. Oceanogr. 130, 205–248 (2015).
Clark, J. R. et al. Marine microplastic debris: a targeted plan for understanding and quantifying interactions with marine life. Front. Ecol. Environ. 14, 317–324 (2016).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotech. 7, 779–786 (2012).
Tenzer, S. et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotech. 8, 772–781 (2013).
Smita, S. et al. Nanoparticles in the environment: assessment using the causal diagram approach. Environ. Health 11, 1 (2012).
Walczyk, D., Bombelli, F. B., Monopoli, M. P., Lynch, I. & Dawson, K. A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768 (2010).
Walkey, C. D. & Chan, W. C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 41, 2780–2799 (2012).
Lundquist, J. J. & Toone, E. J. The cluster glycoside effect. Chem. Rev. 102, 555–578 (2002).
Gibson, C., Turner, I. J., Roberts, C. J. & Lead, J. Quantifying the dimensions of nanoscale organic surface layers in natural waters. Environ. Sci. Technol. 41, 1339–1344 (2007).
Cole, M. et al. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 50, 3239–3246 (2016). Introduces the hypothesis that faecal pellets provide a route for the sinking of microplastics from surface waters to the ocean floor.
Wright, S. L., Rowe, D., Thompson, R. C. & Galloway, T. S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 23, 1031–1033 (2013).
Park, E.-J., Yi, J., Kim, Y., Choi, K. & Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 24, 872–878 (2010).
Khan, F. R., Syberg, K., Shashoua, Y. & Bury, N. R. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ. Pollut. 206, 73–79 (2015).
Fotopoulou, K. N. & Karapanagioti, H. K. Surface properties of beached plastic pellets. Mar. Environ. Res. 81, 70–77 (2012).
Wotton, R. S. The essential role of exopolymers (EPS) in aquatic systems. Oceanogr. Mar. Biol. Annu. Rev. 42, 57–94 (2004).
Charlson, R. J., Lovelock, J. E., Andreae, M. O. & Warren, S. G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326, 655–661 (1987).
Savoca, M. S., Wohlfeil, M. E., Ebeler, S. E. & Nevitt, G. A. Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2 e1600395 (2016).
Nasser, F. & Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteomics 137, 45–51 (2016).
Oberbeckmann, S., Löder, M. G. & Labrenz, M. Marine microplastic-associated biofilms–a review. Environ. Chem. 12, 551–562 (2015).
De Tender, C. A. et al. Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ. Sci. Technol. 49, 9629–9638 (2015).
Foulon, V. et al. Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ. Sci. Technol. 50, 10988–10996 (2016).
Long, M. et al. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 175, 39–46 (2015).
Thiele, S., Fuchs, B. M., Amann, R. & Iversen, M. H. Colonization in the photic zone and subsequent changes during sinking determine bacterial community composition in marine snow. Appl. Environ. Microbiol. 81, 1463–1471 (2015).
Jatt, A. N., Tang, K., Liu, J., Zhang, Z. & Zhang, X.-H. Quorum sensing in marine snow and its possible influence on production of extracellular hydrolytic enzymes in marine snow bacterium Pantoea ananatis B9. FEMS Microbiol. Ecol. 91, 1–13 (2015).
Lusher, A., Welden, N., Sobral, P. & Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods (2016).
Adverse Outcome Pathways, Molecular Screening and Toxicogenomics (OECD, 2016).
Jeong, C.-B. et al. Microplastic size-dependent toxicity, oxidative stress induction, and p-jnk and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 50, 8849–8857 (2016).
Paul-Pont, I. et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 216, 724–737 (2016).
Oliveira, M., Ribeiro, A., Hylland, K. & Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 34, 641–647 (2013).
Rist, S. E. et al. Suspended micro-sized PVC particles impair the performance and decrease survival in the Asian green mussel Perna viridis. Mar. Pollut. Bull. 111, 213–220 (2016).
Ogonowski, M., Schür, C., Jarsén, Å. & Gorokhova, E. The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS ONE 11, e0155063 (2016).
Galloway, T. S. & Lewis, C. N. Marine microplastics spell big problems for future generations. Proc. Natl Acad. Sci. USA 113, 2331–2333 (2016).
Cole, M., Lindeque, P., Fileman, E., Halsband, C. & Galloway, T. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 49, 1130–1137 (2015).
Welden, N. A. C. & Cowie, P. R. Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. Pollut. 214, 859–865 (2016).
Watts, A. J. R., Urbina, M. A., Corr, S., Lewis, C. & Galloway, T. S. Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol. 49, 14597–14604 (2015).
Wright, S., Rowe, D., Thompson, R. C. & Galloway, T. S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 23, 1031–1033 (2013).
Green, D. S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 216, 95–103 (2016).
Cole, M. & Galloway, T. Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ. Sci. Technol. 49, 14625–14632 (2015).
Kaposi, K. L., Mos, B., Kelaher, B. & Dworjanyn, S. A. Ingestion of microplastic has limited impact on a marine larva. Environ. Sci. Technol. 48, 1638–1645 (2013).
Sussarellu, R. et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl Acad. Sci. USA 113, 2430–2435 (2016). Provides data to illustrate multigenerational effects of microplastic in an invertebrate model illustrating potential ecological impact in marine ecosystems.
Ha¨mer, J., Gutow, L., Ko¨hler, A. & Saborowski, R. Fate of microplastics in the marine isopod Idotea emarginata. Environ. Sci. Technol. 48, 13451–13458 (2014).
Blarer, P. & Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res. 23, 23522–23532 (2016).
Lee, K.-W., Shim, W. J., Kwon, O. Y. & Kang, J.-H. Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol. 47, 11278–11283 (2013).
Weis, J. S. Developmental and Behavioural Effects of Marine Pollution (Springer, 2014).
Wong, B. B. & Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673 (2015).
de Sá, L. C., Luís, L. G. & Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 196, 359–362 (2015).
Waloff, N. The mechanisms of humidity reactions of terrestrial isopods. J. Exp. Biol. 18, 8–135 (1941).
Tosetto, L., Brown, C. & Williamson, J. E. Microplastics on beaches: ingestion and behavioural consequences for beachhoppers. Mar. Biol. 163, 199 (2016).
Rehse, S., Kloas, W. & Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 153, 91–99 (2016).
Godin, J.-G. J. & Crossman, S. L. Hunger-dependent predator inspection and foraging behaviours in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav. Ecol. Sociobiol. 34, 359–366 (1994).
Dill, L. M. & Fraser, A. H. Risk of predation and the feeding behavior of juvenile coho salmon (Oncorhynchus kisutch). Behav. Ecol. Sociobiol. 16, 65–71 (1984).
Gotceitas, V. & Godin, J.-G. J. Foraging under the risk of predation in juvenile Atlantic salmon (Salmo salar L.): effects of social status and hunger. Behav. Ecol. Sociobiol. 29, 255–261 (1991).
Houston, A. I. & McNamara, J. M. Models of Adaptive Behaviour: an Approach Based on State. (Cambridge Univ. Press, 1999).
Meadows, P. S., Meadows, A. & Murray, J. M. Biological modifiers of marine benthic seascapes: Their role as ecosystem engineers. Geomorphology 157, 31–48 (2012).
Green, D. S., Boots, B., Sigwart, J., Jiang, S. & Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 208, 426–434 (2016).
Sailley, S. F., Polimene, L., Mitra, A., Atkinson, A. & Allen, J. I. Impact of zooplankton food selectivity on plankton dynamics and nutrient cycling. J. Plankton Res. 37, 519–529 (2015).
Desforges, J.-P. W., Galbraith, M. & Ross, P. S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 69, 320–330 (2015). First report of ingestion of microplastics in situ by zooplankton indicating that species at lower trophic levels can mistake plastic for food. This highlights the potential risks to higher trophic level species.
Buesseler, K. O. et al. Revisiting carbon flux through the ocean's twilight zone. Science 316, 567–570 (2007).
Small, L., Fowler, S. & Ünlü, M. Sinking rates of natural copepod fecal pellets. Mar. Biol. 51, 233–241 (1979).
Giering, S. L. et al. Reconciliation of the carbon budget in the ocean/'s twilight zone. Nature 507, 480–483 (2014).
Report of the First Session of the INC for an International Legally Binding Instrument for Implementing International Action on certain Persistent Organic Pollutants (POPs) (UNEP, 1998).
Diamond, M. L. et al. Exploring the planetary boundary for chemical pollution. Environ. Int. 78, 8–15 (2015).
Rockström, J. et al. A safe operating space for humanity. Nature 461, 472–475 (2009).
Oil Tanker Spill Statistics (ITOPF, 2016).
Sanderson, K. It's not easy being green. Nature 469, 18–20 (2011).
Guide to the Business of Chemistry, American Chemical Council Facts and Figs 2011 (CEFIC, 2011).
Global Plastic Production from 1950 to 2015 (STATISTA, 2016).
Scheringer, M. Characterization of the environmental distribution behavior of organic chemicals by means of persistence and spatial range. Environ. Sci. Technol. 31, 2891–2897 (1997).
Scheringer, M. Persistence and spatial range as endpoints of an exposure-based assessment of organic chemicals. Environ. Sci. Technol. 30, 1652–1659 (1996).
Persson, L. M. et al. Confronting unknown planetary boundary threats from chemical pollution. Environ. Sci. Technol. 47, 12619–12622 (2013).
Acknowledgements
Funding was provided by the Natural Environment Research Council (grants NE/L007010 and NE/N006178) and European Union FP7 (grant 308370). We gratefully acknowledge helpful discussions with colleagues, including R. Lohmann (University of Rhode Island) for discussions on persistent organic pollutants.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Galloway, T., Cole, M. & Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1, 0116 (2017). https://doi.org/10.1038/s41559-017-0116
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0116
This article is cited by
-
The soil plastisphere
Nature Reviews Microbiology (2024)
-
Nominally identical microplastic models differ greatly in their particle-cell interactions
Nature Communications (2024)
-
Microplastics and nanoplastics size distribution in farmed mussel tissues
Communications Earth & Environment (2024)
-
Occurrence of microplastics in fish gastrointestinal tracts belongs to different feeding habits from the Bangladesh coast of the Bay of Bengal
Environmental Science and Pollution Research (2024)
-
Exploring the blindspot: The soil plastisphere
Soil Ecology Letters (2024)