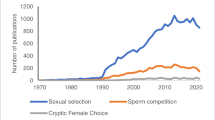
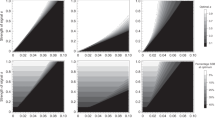
Abstract
Sexual conflict is the divergence of evolutionary interests between the sexes. A neglected aspect of sexual conflict theory is that the conflict often occurs within the female’s body, which can lead to a power asymmetry between the sexes. In particular, the female may often be able to respond flexibly to the actions of the male, and so exhibits plasticity. Here, we consider the implications of female plasticity, and find that it tends to result in lower levels of sexual conflict. We then relate our results to a comparison of pre- versus post-copulatory sexual conflict, and we also show that this asymmetry between males and females reduces the likelihood of runaway selection, preventing co-evolutionary arms races. Finally, we discuss our results in the context of the evolution of adaptive harm and sexual conflict when there are direct benefits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Trivers, R. L. in Sexual Selection and the Descent of Man (ed. Campbell, B. ) 136–179 (Aladine, 1972).
Parker, G. in Sexual Selection and Reproductive Competition in Insects (eds Blum, M. & Blum, N. A. ) 123–166 (Academic, 1979).
Chapman, T., Arnqvist, G., Bangham, J. & Rowe, L. Sexual conflict. Trends Ecol. Evol. 18, 41–47 (2003).
Arnqvist, G. & Rowe, L. Sexual Conflict (Princeton Univ. Press, 2005).
Arnqvist, G. & Rowe, L. Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789 (2002).
Sirot, L. K., Wong, A., Chapman, T. & Wolfner, M. F. Sexual conflict and seminal fluid proteins: a dynamic landscape of sexual interactions. Cold Spring Harb. Perspect. Biol. 7, 1–24 (2014).
Civetta, A. & Clark, A. G. Correlated effects of sperm competition and postmating female mortality. Proc. Natl Acad. Sci. USA 97, 13162–13165 (2000).
Wigby, S. & Chapman, T. Sex peptide causes mating costs in female Drosophila melanogaster . Curr. Biol. 15, 316–321 (2005).
Fricke, C., Bretman, A. & Chapman, T. Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster . J. Evol. Biol. 23, 157–165 (2010).
Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 (1995).
Rice, W. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234 (1996).
Abbott, J. K., Innocenti, P., Chippindale, A. K. & Morrow, E. H. Epigenetics and sex-specific fitness: an experimental test using male-limited evolution in Drosophila melanogaster . PLoS ONE 8, e70493 (2013).
Gavrilets, S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889 (2000).
Gavrilets, S., Arnqvist, G. & Friberg, U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. Lond. B 268, 531–539 (2001).
Gavrilets, S. & Waxman, D. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA 99, 10533–10538 (2002).
Rowe, L., Cameron, E. & Day, T. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 165, S5–S18 (2005).
Clutton-Brock, T. H. & Parker, G. A. Sexual coercion in animal societies. Anim. Behav. 49, 1345–1365 (1995).
Eberhard, W. G. Female Control: Sexual Selection by Cryptic Female Choice (Princeton Univ. Press, 1996).
Parker, G. A. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235–259 (2006).
Holland, B. & Rice, W. R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7 (1998).
Chapman, T. Evolutionary conflicts of interest between males and females. Curr. Biol. 16, R744–R754 (2006).
McNamara, J. M. & Dall, S. R. X. Information is a fitness enhancing resource. Oikos 119, 231–236 (2010).
McNamara, J. M., Gasson, C. E. & Houston, A. I. Incorporating rules for responding into evolutionary games. Nature 401, 368–371 (1999).
Lupold, S. et al. How sexual selection can drive the evolution of costly sperm ornamentation. Nature 533, 535–538 (2016).
Schneider, M. R., Mangels, R. & Dean, M. D. The molecular basis and reproductive function(s) of copulatory plugs. Mol. Reprod. Dev. 83, 755–767 (2016).
Koprowski, J. L. Removal of copulatory plugs by female tree squirrels. J. Mammal. 73, 572–576 (1992).
Kelleher, E. S. & Pennington, J. E. Protease gene duplication and proteolytic activity in Drosophila female reproductive tracts. Mol. Biol. Evol. 26, 2125–2134 (2009).
Dean, M. D. et al. Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics 12, 306 (2011).
Mangels, R. et al. Genetic and phenotypic influences on copulatory plug survival in mice. Heredity 115, 496–502 (2015).
Chapman, T. et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA 100, 9923–9928 (2003).
Ravi Ram, K. & Wolfner, M. F. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47, 427–445 (2007).
Pilpel, N., Nezer, I., Applebaum, S. W. & Heifetz, Y. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem. Mol. Biol. 38, 320–330 (2008).
Prokupek, A. M., Kachman, S. D., Ladunga, I. & Harshman, L. G. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster . Insect Mol. Biol. 18, 465–475 (2009).
Johnstone, R. A. & Keller, L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am. Nat. 156, 368–377 (2000).
Lessells, C. M. Why are males bad for females? Models for the evolution of damaging male mating behavior. Am. Nat. 165, S46–S63 (2005).
Morrow, E., Arnqvist, G. & Pitnick, S. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 14, 802–806 (2003).
Vahed, K. The function of nuptial feeding in insects: a review of empirical studies. Biol. Rev. 73, 43–78 (1998).
Arnqvist, G. & Nilsson, T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 (2000).
Karlsson, B. Resource allocation and mating systems in butterflies. Evolution 49, 955–961 (1995).
Pike, R. K., McNamara, J. M. & Houston, A. I. A general expression for the reproductive value of information. Behav. Ecol. (in the press).
Acknowledgements
We thank L. Rowe for comments on an earlier version of the manuscript. Funding support was provided through an NSERC scholarship to D.V.M. and an NSERC grant to T.D.
Author information
Authors and Affiliations
Contributions
D.V.M. and T.D. formulated the research question, designed and analysed the model, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary modelling; Supplementary Figures 1,2 (PDF 235 kb)
Rights and permissions
About this article
Cite this article
McLeod, D., Day, T. Female plasticity tends to reduce sexual conflict. Nat Ecol Evol 1, 0054 (2017). https://doi.org/10.1038/s41559-016-0054
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-016-0054