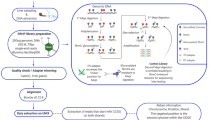
Abstract
Epigenetic inheritance is a potential mechanism by which the environment in one generation can influence the performance of future generations1. Rapid climate change threatens the survival of many organisms; however, recent studies show that some species can adjust to climate-related stress when both parents and their offspring experience the same environmental change2,3. Whether such transgenerational acclimation could have an epigenetic basis is unknown. Here, by sequencing the liver genome, methylomes and transcriptomes of the coral reef fish, Acanthochromis polyacanthus, exposed to current day (+0 °C) or future ocean temperatures (+3 °C) for one generation, two generations and incrementally across generations, we identified 2,467 differentially methylated regions (DMRs) and 1,870 associated genes that respond to higher temperatures within and between generations. Of these genes, 193 were significantly correlated to the transgenerationally acclimating phenotypic trait, aerobic scope, with functions in insulin response, energy homeostasis, mitochondrial activity, oxygen consumption and angiogenesis. These genes may therefore play a key role in restoring performance across generations in fish exposed to increased temperatures associated with climate change. Our study is the first to demonstrate a possible association between DNA methylation and transgenerational acclimation to climate change in a vertebrate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jablonka, E. & Raz, G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 (2009).
Donelson, J., Munday, P., McCormick, M. & Pitcher, C. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32 (2012).
Salinas, S. & Munch, S. B. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163 (2012).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500 (2013).
Chevin, L. M., Lande, R. & Mace, G. M. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 (2010).
Bonduriansky, R., Crean, A. J. & Day, T. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 5, 192–201 (2012).
Daxinger, L. & Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 13, 153–162 (2012).
Ng, S. F. et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010).
Veilleux, H. D. et al. Molecular processes of transgenerational acclimation to a warming ocean. Nat. Clim. Change 5, 1074–1078 (2015).
Donelson, J. M., Wong, M., Booth, D. J. & Munday, P. L. Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol. Appl. 9, 1072–1081 (2016).
Rui, L. Energy metabolism in the liver. Compr. Physiol. 4, 177–197 (2014).
Das, J. The role of mitochondrial respiration in physiological and evolutionary adaptation. Bioessays 28, 890–901 (2006).
Kashio, M. & Tominaga, M. The TRPM2 channel: A thermo-sensitive metabolic sensor. Channels 11, 426–433 (2017).
Kang, H. W., Wei, J. & Cohen, D. E. PC-TP/StARD2: of membranes and metabolism. Trends Endocrinol. Metab. 21, 449–456 (2010).
Puri, V. et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl Acad. Sci. USA 105, 7833–7838 (2008).
Zhou, Z. et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 35, 49–56 (2003).
Portner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Fraisl, P., Mazzone, M., Schmidt, T. & Carmeliet, P. Regulation of angiogenesis by oxygen and metabolism. Dev. Cell 16, 167–179 (2009).
Laramee, M. et al. The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J. Biol. Chem. 282, 7758–7769 (2007).
Chao, W. & D’Amore, P. A. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth F. Rev. 19, 111–120 (2008).
de Vries, S. et al. Identification of DEAD-box RNA helicase 6 (DDX6) as a cellular modulator of vascular endothelial growth factor expression under hypoxia. J. Biol. Chem. 288, 5815–5827 (2013).
Bard-Chapeau, E. A. et al. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat. Med. 11, 567–571 (2005).
Ding, G. L. et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 61, 1133–1142 (2012).
Vangeel, E. B. et al. DNA methylation in imprinted genes IGF2 and GNASXL is associated with prenatal maternal stress. Genes Brain Behav. 14, 573–582 (2015).
Gabillard, J. C., Rescan, P. Y., Fauconneau, B., Weil, C. & Le Bail, P. Y. Effect of temperature on gene expression of the Gh/Igf system during embryonic development in rainbow trout (Oncorhynchus mykiss). J. Exp. Zool. A 298, 134–142 (2003).
Xie, B., Zhang, L., Zheng, K. & Luo, C. The evolutionary foundation of genomic imprinting in lower vertebrates. Chin. Sci. Bull. 54, 1354 (2009).
Lou, S. et al. Whole-genome bisulfite sequencing of multiple individuals reveals complementary roles of promoter and gene body methylation in transcriptional regulation. Genome Biol. 15, 408 (2014).
Hu, W. et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl Acad. Sci. USA 107, 7455–7460 (2010).
Vukotic, M. et al. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 15, 336–347 (2012).
Chung, D. J. & Schulte, P. M. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 218, 1621–1631 (2015).
Rangwala, S. M. et al. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J. Biol. Chem. 285, 22619–22629 (2010).
van den Hoogenhof, M. M., Pinto, Y. M. & Creemers, E. E. RNA splicing: regulation and dysregulation in the heart. Circ. Res 118, 454–468 (2016).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual 3rd edn (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2001).
Simpson, J. T. et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 (2009).
Boetzer, M., Henkel, C. V., Jansen, H. J., Butler, D. & Pirovano, W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27, 578–579 (2011).
Parra, G., Bradnam, K. & Korf, I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 23, 1061–1067 (2007).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Quinlan, A. R. BEDTools: the Swiss-army tool for genome feature analysis. Curr. Protoc. Bioinform. 47, 11–34 (2014). 11 12.
Robertson, G. et al. De novo assembly and analysis of RNA-seq data. Nat. Methods 7, 909–912 (2010).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Holt, C. & Yandell, M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 12, 491 (2011).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Boutet, E., Lieberherr, D., Tognolli, M., Schneider, M. & Bairoch, A. UniProtKB/Swiss-Prot. Methods Mol. Biol. 406, 89–112 (2007).
Korf, I. Gene finding in novel genomes. BMC Bioinform. 5, 59 (2004).
Stanke, M., Steinkamp, R., Waack, S. & Morgenstern, B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 32, W309–W312 (2004).
Tarailo-Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform. 25, 4.10.1–4.10.14 (2009).
Li, W. et al. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 43, W580–W584 (2015).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Krueger F. Trim Galore! v. 0.4 (Babraham Bioinformatics, 2015); http://www.bioinformatics.babraham.ac.uk/projects/trim_galore
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572 (2011).
Akalin, A. et al. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 13, R87 (2012).
Warnes, G. R. et al. gplots: various R programming tools for plotting data R package v. 3.0.1 (R Foundation, 2016); http://CRAN.R-project.org/package=gplots
De Leeuw, J. & Mair, P. Multidimensional scaling using majorization: SMACOF in R. J. Stat. Softw. 31, i03 (2009).
Nordhausen, K., Sirkia, S., Oja, H. & Tyler, D. ICSNP: Tools for Multivariate Nonparametrics R package v. 1.1-0. (R Foundation, 2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
This study was supported by the Competitive Research Funds OCRF-2014-CRG3-62140408 from the King Abdullah University of Science and Technology. This project was completed under JCU Ethics A1233 and A1415. T.Ryu acknowledges the support from the APEC Climate Center. P.L.M. was supported by the Australian Research Council (ARC) and P.L.M., H.D.V. and J.M.D. were supported by the ARC Centre of Excellence for Coral Reef Studies. We thank C. Ortiz Alvarez and E. J. Steinig (James Cook University) for assisting genomic DNA extraction for methylome sequencing. Figures were enhanced by I. Gromicho, scientific illustrator at King Abdullah University of Science and Technology (KAUST).
Author information
Authors and Affiliations
Contributions
J.M.D. managed the fish rearing experiments and performed metabolism experiments. H.D.V. prepared samples for sequencing. H.D.V. extracted nucleic acids for genome and transcriptiome. T.Ryu extracted nucleic acids for methylome sequencing. T.Ryu, H.D.V. and J.M.D. selected samples for sequencing. T.Ryu designed and performed the computational analysis. T.Ryu and H.D.V. interpreted the results. T.Ryu, T.Ravasi, P.L.M., H.D.V. and J.M.D. wrote the manuscript. T.Ravasi and P.L.M. supervised the overall project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1 & 2, Supplementary Tables 1–3, 5, 6, 8, 9
Supplementary Table 4
Genomic coordinates of identified differentially methylated regions (DMRs)
Supplementary Table 7
Differentially methylated and net aerobic scope (NAS)-correlated gene information
Rights and permissions
About this article
Cite this article
Ryu, T., Veilleux, H.D., Donelson, J.M. et al. The epigenetic landscape of transgenerational acclimation to ocean warming. Nature Clim Change 8, 504–509 (2018). https://doi.org/10.1038/s41558-018-0159-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-018-0159-0
This article is cited by
-
Heatwave resilience of juvenile white sturgeon is associated with epigenetic and transcriptional alterations
Scientific Reports (2023)
-
Epigenetic effects associated with salmonid supplementation and domestication
Environmental Biology of Fishes (2023)
-
Paternal hypoxia exposure primes offspring for increased hypoxia resistance
BMC Biology (2022)
-
Leveraging transcriptome and epigenome landscapes to infer regulatory networks during the onset of sexual maturation
BMC Genomics (2022)
-
Long term environmental variability modulates the epigenetics of maternal traits of kelp crabs in the coast of Chile
Scientific Reports (2022)