Abstract
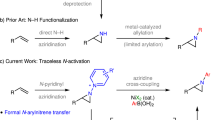
N–N linkages are found in many natural compounds and endow fascinating structural and functional properties. In comparison to the myriad methods for the construction of C–N bonds, chemistry for N–N coupling, especially in an intermolecular fashion, remains underdeveloped. Here, we report a nitrene-mediated intermolecular N–N coupling of dioxazolones and arylamines under iridium or iron catalysis. These reactions offer a simple and efficient method for the synthesis of various hydrazides from readily available carboxylic acid and amine precursors. Although the Ir-catalysed conditions usually give higher N–N coupling yield than the Fe-catalysed conditions, the reactions of sterically more demanding dioxazolones derived from α-substituted carboxylic acids work much better under the Fe-catalysed conditions. Mechanistic studies revealed that the nitrogen atom of Ir acyl nitrene intermediates has strong electrophilicity and can undergo nucleophilic attack with arylamines with the assistance of Cl···HN hydrogen bonding to form the N–N bond with high efficiency and chemoselectivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The X-ray crystallographic data for compounds 10c and [Ir]-TolNH2 have been deposited in the Cambridge Crystallographic Data Centre with CCDC nos. 1952123 and 1956819, respectively, and can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Hili, R. & Yudin, A. K. Making carbon–nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006).
Ricci, A. Amino Group Chemistry: from Synthesis to the Life Sciences (Wiley, 2008).
Blair, L. M. & Sperry, J. Natural products containing a nitrogen–nitrogen bond. J. Nat. Prod. 76, 794–812 (2013).
Waldman, A. J., Ng, T. L., Wang, P. & Balskus, E. P. Heteroatom–heteroatom bond formation in natural product biosynthesis. Chem. Rev. 117, 5784–5863 (2017).
Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 30, 205–213 (2001).
Guo, Q. H. & Lu, Z. Recent advances in nitrogen–nitrogen bond formation. Synthesis 49, 3835–3847 (2017).
Wolter, M., Klapars, A. & Buchwald, S. L. Synthesis of N-aryl hydrazides by copper-catalyzed coupling of hydrazides with aryl iodides. Org. Lett. 3, 3803–3805 (2001).
Evans, D. A. & Johnson, D. S. Catalytic enantioselective amination of enolsilanes using C2-symmetric copper(ii) complexes as chiral Lewis acids. Org. Lett. 1, 595–598 (1999).
Vidal, J., Hannachi, J. C., Hourdin, G., Mulatier, J. C. & Collet, A. N-Boc-3-trichloromethyloxaziridine: a new, powerful reagent for electrophilic amination. Tetrahedron Lett. 39, 8845–8848 (1998).
Rosen, B. R., Werner, E. W., O’Brien, A. G. & Baran, P. S. Total synthesis of dixiamycin B by electrochemical oxidation. J. Am. Chem. Soc. 136, 5571–5574 (2014).
Ryan, M. C., Martinelli, J. R. & Stahl, S. S. Cu-catalyzed aerobic oxidative N–N coupling of carbazoles and diarylamines including selective cross-coupling. J. Am. Chem. Soc. 140, 9074–9077 (2018).
Ryan, M. C. et al. Mechanistic insights into copper-catalyzed aerobic oxidative coupling of N–N bonds. Chem. Sci. 11, 1170–1175 (2020).
Stokes, B. J., Vogel, C. V., Urnezis, L. K., Pan, M. & Driver, T. G. Intramolecular Fe(ii)-catalyzed N–O or N–N bond formation from aryl azides. Org. Lett. 12, 2884–2887 (2010).
Neumann, J. J., Suri, M. & Glorius, F. Efficient synthesis of pyrazoles: oxidative C–C/N–N bond-formation cascade. Angew. Chem. Int. Ed. 49, 7790–7794 (2010).
Zhang, Y. et al. Fe(iii)-catalyzed aerobic intramolecular N–N coupling of aliphatic azides with amines. Org. Lett. 21, 4960–4965 (2019).
Diccianni, J. B., Hu, C. H. & Diao, T. N. N–N bond forming reductive elimination via a mixed-valent nickel(ii)–nickel(iii) intermediate. Angew. Chem. Int. Ed. 55, 7534–7538 (2016).
Maestre, L. et al. Functional-group-tolerant, silver-catalyzed N–N bond formation by nitrene transfer to amines. J. Am. Chem. Soc. 139, 2216–2223 (2017).
Li, J. et al. Erratum: Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate. Nat. Chem. 5, 634–634 (2013).
Kono, M., Harada, S. & Nemoto, T. Chemoselective intramolecular formal insertion reaction of Rh-nitrenes into an amide bond over C–H insertion. Chem. Eur. J. 25, 3119–3124 (2019).
Dehghany, M., Eshon, J., Roberts, J. M. & Schomaker, J. M. in Silver Catalysis in Organic Synthesis (eds Li, C. J. & Bi, X. H.) Ch. 8, 439–532 (Wiley, 2019).
Lang, K., Torker, S., Wojtas, L. & Zhang, X. P. Asymmetric induction and enantiodivergence in catalytic radical C–H amination via enantiodifferentiative H-atom abstraction and stereoretentive radical substitution. J. Am. Chem. Soc. 141, 12388–12396 (2019).
Ju, M. et al. Tunable catalyst-controlled syntheses of β- and γ-amino alcohols enabled by silver-catalysed nitrene transfer. Nat. Catal. 2, 899–908 (2019).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Park, Y., Heo, J., Baik, M.-H. & Chang, S. Why is the Ir(iii)-mediated amido transfer much faster than the Rh(iii)-mediated reaction? A combined experimental and computational study. J. Am. Chem. Soc. 138, 14020–14029 (2016).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism and applications. Chem. Rev. 117, 9247–9301 (2017).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Park, Y., Park, K. T., Kim, J. G. & Chang, S. Mechanistic studies on the Rh(iii)-mediated amido transfer process leading to robust C–H amination with a new type of amidating reagent. J. Am. Chem. Soc. 137, 4534–4542 (2015).
Dequirez, G., Pons, V. & Dauban, P. Nitrene chemistry in organic synthesis: still in its infancy? Angew. Chem. Int. Ed. 51, 7384–7395 (2012).
Shimbayashi, T., Sasakura, K., Eguchi, A., Okamoto, K. & Ohe, K. Recent progress on cyclic nitrenoid precursors in transition-metal-catalyzed nitrene-transfer reactions. Chem. Eur. J. 25, 3156–3180 (2019).
van Vliet, K. M. & de Bruin, B. Dioxazolones: stable substrates for the catalytic transfer of acyl nitrenes. ACS Catal. 10, 4751–4769 (2020).
Sauer, J. & Mayer, K. K. Thermolyse und photolyse von 3-subtituierten Δ2-1.4.2-dioxazolinonen-(5), Δ2-1.4.2-dioxazolin-thionen-(5) und 4-substituierten Δ3-1.2.5.3-thiadioxazolin-s-oxiden. Tetrahedron Lett. 9, 319–324 (1968).
Bizet, V., Buglioni, L. & Bolm, C. Light-induced ruthenium-catalyzed nitrene transfer reactions: a photochemical approach towards N-acyl sulfimides and sulfoximines. Angew. Chem. Int. Ed. 53, 5639–5642 (2014).
Lebel, H., Piras, H. & Bartholomeus, J. Rhodium-catalyzed stereoselective amination of thioethers with N-mesyloxycarbamates: DMAP and bis(DMAP)CH2Cl2 as key additives. Angew. Chem. Int. Ed. 53, 7300–7304 (2014).
van Vliet, K. M. et al. Efficient copper-catalyzed multicomponent synthesis of N-acyl amidines via acyl nitrenes. J. Am. Chem. Soc. 141, 15240–15249 (2019).
Park, Y. & Chang, S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat. Catal. 2, 219–227 (2019).
Wang, H. et al. Iridium-catalyzed enantioselective C(sp3)–H amidation controlled by attractive noncovalent interactions. J. Am. Chem. Soc. 141, 7194–7201 (2019).
Xing, Q., Chan, C. M., Yeung, Y. W. & Yu, W. Y. Ruthenium(ii)-catalyzed enantioselective γ-lactams formation by intramolecular C–H amidation of 1,4,2-dioxazol-5-ones. J. Am. Chem. Soc. 141, 3849–3853 (2019).
Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Wang, H., Tang, G. & Li, X. Rhodium(iii)-catalyzed amidation of unactivated C(sp3)–H bonds. Angew. Chem. Int. Ed. 54, 13049–13052 (2015).
Mei, R., Loup, J. & Ackermann, L. Oxazolinyl-assisted C–H amidation by cobalt(iii) catalysis. ACS Catal. 6, 793–797 (2016).
Lei, H. & Rovis, T. Ir-catalyzed intermolecular branch-selective allylic C–H amidation of unactivated terminal olefins. J. Am. Chem. Soc. 141, 2268–2273 (2019).
Knecht, T., Mondal, S., Ye, J. H., Das, M. & Glorius, F. Intermolecular, branch-selective, and redox-neutral Cp*Ir(iii)-catalyzed allylic C–H amidation. Angew. Chem. Int. Ed. 58, 7117–7121 (2019).
Scamp, R. J., deRamon, E., Paulson, E. K., Miller, S. J. & Ellman, J. A. Co(iii)-catalyzed C–H amidation of dehydroalanine for the site-selective structural diversification of thiostrepton. Angew. Chem. Int. Ed. 59, 890–895 (2020).
Zhou, Z. et al. Non-C2-symmetric chiral-at-ruthenium catalyst for highly efficient enantioselective intramolecular C(sp3)–H amidation. J. Am. Chem. Soc. 141, 19048–19057 (2019).
Burman, J. S., Harris, R. J., B. Farr, C. M., Bacsa, J. & Blakey, S. B. Rh(iii) and Ir(iii)Cp* complexes provide complementary regioselectivity profiles in intermolecular allylic C–H amidation reactions. ACS Catal. 9, 5474–5479 (2019).
Tan, P. W., Mak, A. M., Sullivan, M. B., Dixon, D. J. & Seayad, J. Thioamide-directed cobalt(iii)-catalyzed selective amidation of C(sp3)−H bonds. Angew. Chem. Int. Ed. 56, 16550–16554 (2017).
Fukagawa, S., Kojima, M., Yoshino, T. & Matsunaga, S. Catalytic enantioselective methylene C(sp3)–H amidation of 8-alkylquinolines using a Cp*Rhiii/chiral carboxylic acid system. Angew. Chem. Int. Ed. 58, 18154–18158 (2019).
Dube, P. et al. Carbonyldiimidazole-mediated Lossen rearrangement. Org. Lett. 11, 5622–5625 (2009).
Legnani, L. & Morandi, B. Direct catalytic synthesis of unprotected 2-amino-1-phenylethanols from alkenes by using iron(ii) phthalocyanine. Angew. Chem. Int. Ed. 55, 2248–2251 (2016).
Liu, G.-S., Zhang, Y.-Q., Yuan, Y.-A. & Xu, H. Iron(ii)-catalyzed intramolecular aminohydroxylation of olefins with functionalized hydroxylamines. J. Am. Chem. Soc. 135, 3343–3346 (2013).
Zhang, Y.-Q., Yuan, Y.-A., Liu, G.-S. & Xu, H. Iron(ii)-catalyzed asymmetric intramolecular aminohydroxylation of indoles. Org. Lett. 15, 3910–3913 (2013).
Zhang, Y. et al. Fe(iii)-catalyzed aerobic intramolecular N−N coupling of aliphatic azides with amines. Org. Lett. 21, 4960–4965 (2019).
Yu, H., Li, Z. & Bolm, C. Three-dimensional heterocycles by iron-catalyzed ring-closing sulfoxide imidation. Angew. Chem. Int. Ed. 57, 12053–12056 (2018).
Yao, J., Feng, R., Lin, C., Liu, Z. & Zhang, Y. Synthesis of 2,3-dihydro-1H-indazoles by Rh(iii)-catalyzed C–H cleavage of arylhydrazines. Org. Biomol. Chem. 12, 5469–5476 (2014).
Han, S. et al. Rh(iii)-catalyzed oxidative coupling of 1,2-disubstituted arylhydrazines and olefins: a new strategy for 2,3-dihydro-1H-indazoles. Org. Lett. 16, 2494–2497 (2014).
Zhan, F. & Liang, G. Formation of enehydrazine intermediates through coupling of phenylhydrazines with vinyl halides: entry into the Fischer indole synthesis. Angew. Chem. Int. Ed. 52, 1266–1269 (2013).
Patureau, F. W. & Glorius, F. Oxidizing directing groups enable efficient and innovative C–H activation reactions. Angew. Chem. Int. Ed. 50, 1977–1979 (2011).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Rummelt, S. M., Radkowski, K., Rosca, D. A. & Furstner, A. Interligand interactions dictate the regioselectivity of trans-hydrometalations and related reactions catalyzed by [Cp*RuCl]. hydrogen bonding to a chloride ligand as a steering principle in catalysis. J. Am. Chem. Soc. 137, 5506–5519 (2015).
Kuijpers, P. F., van der Vlugt, J. I., Schneider, S. & de Bruin, B. Nitrene radical intermediates in catalytic synthesis. Chem. Eur. J. 23, 13819–13829 (2017).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Shing, K.-P. et al. N-heterocyclic carbene iron(iii) porphyrin-catalyzed intramolecular C(sp3)–H amination of alkyl azides. Angew. Chem. Int. Ed. 57, 11947–11951 (2018).
Wang, P. & Deng, L. Recent advances in iron-catalyzed C–H bond amination via iron imido intermediate. Chin. J. Chem. 36, 1222–1240 (2018).
Acknowledgements
G.C. acknowledges financial support from grants NSFC-21725204, NSFC-21672105, NSFC-21421062, NSFC-21901127 and NCC2020FH02, the China Postdoctoral Science Foundation (2018M640225, 2019T120179) and Laviana Pharma for the experimental part of this work. S.C. thanks the Institute for Basic Science (IBS-R010-D1) in the Republic of Korea for financial support.
Author information
Authors and Affiliations
Contributions
H.W. formulated the initial ideas for this work, carried out most of the reaction optimization and structural determination of products studies, and prepared the Supplementary Information. F.S. helped with the development of reaction conditions and expansion of substrate scope. S.Z., Z.B. and D.C. helped with expanding the substrate scope. H.J. and H.W. conducted the computational studies and prepared part of the manuscript and Supplementary Information. S.C. supervised the computational studies and edited the manuscript. G.H. supervised experimental studies. G.C. supervised the project, coordinated with S.C. on computational studies, and prepared most of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25 and Tables 1–19. Detailed synthetic procedures, compound characterization, NMR spectra, X-ray crystallographic data and computational details.
Supplementary Data 1
The single point energy output files.
Supplementary Data 1
Crystallographic data for compound 10c. CCDC reference 1952123.
Supplementary Data 2
Crystallographic data for compound [Ir]-TolNH2. CCDC reference 1956819.
Supplementary Data 1
Structure factors file for compound [Ir]-TolNH2. CCDC reference 1956819.
Rights and permissions
About this article
Cite this article
Wang, H., Jung, H., Song, F. et al. Nitrene-mediated intermolecular N–N coupling for efficient synthesis of hydrazides. Nat. Chem. 13, 378–385 (2021). https://doi.org/10.1038/s41557-021-00650-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00650-0
This article is cited by
-
Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
Nature Communications (2024)
-
Synthesis of N-acyl sulfenamides via copper catalysis and their use as S-sulfenylating reagents of thiols
Nature Communications (2022)