Abstract
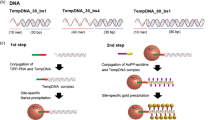
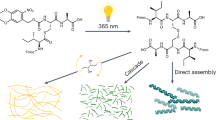
Aqueous compatible supramolecular materials hold promise for applications in environmental remediation, energy harvesting and biomedicine. One remaining challenge is to actively select a target structure from a multitude of possible options, in response to chemical signals, while maintaining constant, physiological conditions. Here, we demonstrate the use of amino acids to actively decorate a self-assembling core molecule in situ, thereby controlling its amphiphilicity and consequent mode of assembly. The core molecule is the organic semiconductor naphthalene diimide, functionalized with D- and L- tyrosine methyl esters as competing reactive sites. In the presence of α-chymotrypsin and a selected encoding amino acid, kinetic competition between ester hydrolysis and amidation results in covalent or non-covalent amino acid incorporation, and variable supramolecular self-assembly pathways. Taking advantage of the semiconducting nature of the naphthalene diimide core, electronic wires could be formed and subsequently degraded, giving rise to temporally regulated electro-conductivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Webber, M. J., Appel, E. A., Meijer, E. W. & Langer, R. Supramolecular biomaterials. Nat. Mater. 15, 13–26 (2016).
Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotech. 21, 1171–1178 (2003).
Campbell, V. E. et al. Cascading transformations within a dynamic self-assembled system. Nat. Chem. 2, 684–687 (2010).
Lehn, J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 36, 151–160 (2007).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Zhang, W. et al. Supramolecular linear heterojunction composed of graphite-like semiconducting nanotubular segments. Science 334, 340–343 (2011).
Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Tantakitti, F. et al. Energy landscapes and functions of supramolecular systems. Nat. Mater. 15, 469–476 (2016).
Maiti, S., Fortunati, I., Ferrante, C., Scrimin, P. & Prins, L. J. Dissipative self-assembly of vesicular nanoreactors. Nat. Chem. 8, 725–731 (2016).
Ashkenasy, G., Hermans, T. M., Otto, S. & Taylor, A. F. Systems chemistry. Chem. Soc. Rev. 46, 2543–2554 (2017).
Heuser, T., Steppert, A.-K., Molano Lopez, C., Zhu, B. & Walther, A. Generic concept to program the time domain of self-assemblies with a self-regulation mechanism. Nano Lett. 15, 2213–2219 (2015).
Debnath, S., Roy, S. & Ulijn, R. V. Peptide nanofibers with dynamic instability through nonequilibrium biocatalytic assembly. J. Am. Chem. Soc. 135, 16789–16792 (2013).
Dhiman, S., Jain, A. & George, S. J. Transient helicity: fuel-driven temporal control over conformational switching in a supramolecular polymer. Angew. Chem. Int. Ed. 56, 1329–1333 (2017).
Sorrenti, A., Leira-Iglesias, J., Sato, A. & Hermans, T. M. Non-equilibrium steady states in supramolecular polymerization. Nat. Commun. 8, 15899-15906 (2017).
Semenov, S. N. et al. Rational design of functional and tunable oscillating enzymatic networks. Nat. Chem. 7, 160–165 (2015).
Epstein, I. R. & Xu, B. Reaction–diffusion processes at the nano- and microscales. Nat. Nanotechnol. 11, 312–319 (2016).
Carnall, J. M. A. et al. Mechanosensitive self-replication driven by self-organization. Science 327, 1502–1506 (2010).
Chen, C. et al. Design of multi-phase dynamic chemical networks. Nat. Chem. 9, 799–804 (2017).
Sadownik, J. W., Mattia, E., Nowak, P. & Otto, S. Diversification of self-replicating molecules. Nat. Chem. 8, 264–269 (2016).
Feng, Z., Wang, H., Zhou, R., Li, J. & Xu, B. Enzyme-instructed assembly and disassembly processes for targeting downregulation in cancer cells. J. Am. Chem. Soc. 139, 3950–3953 (2017).
Grzybowski, B. A. & Huck, W. T. S. The nanotechnology of life-inspired systems. Nat. Nanotechnol. 11, 585–592 (2016).
Mattia, E. & Otto, S. Supramolecular systems chemistry. Nat. Nanotechnol. 10, 111–119 (2015).
Ghadiri, M. R., Granja, J. R., Milligan, R. A., McRee, D. E. & Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 366, 324–327 (1993).
Reches, M. & Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 300, 625–627 (2003).
Du, X., Zhou, J. & Shi, J. & Xu, B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem. Rev. 115, 13165–13307 (2015).
Fleming, S. & Ulijn, R. V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 43, 8150–8177 (2014).
Gazit, E. Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 36, 1263–1269 (2007).
Draper, E. R. & Adams, D. J. Low-molecular-weight gels: the state of the art. Chem 3, 390–410 (2017).
Sanders, A. M., Magnanelli, T. J., Bragg, A. E. & Tovar, J. D. Photoinduced electron transfer within supramolecular donor–acceptor peptide nanostructures under aqueous conditions. J. Am. Chem. Soc. 138, 3362–3370 (2016).
Draper, E. R. et al. Air-stable photoconductive films formed from perylene bisimide gelators. J. Mater. Chem. C 2, 5570–5575 (2014).
Faramarzi, V. et al. Light-triggered self-construction of supramolecular organic nanowires as metallic interconnects. Nat. Chem. 4, 485–490 (2012).
Xu, H. et al. An investigation of the conductivity of peptide nanotube networks prepared by enzyme-triggered self-assembly. Nanoscale 2, 960–966 (2010).
Nalluri, S. K. M., Berdugo, C., Javid, N., Frederix, P. W. J. M. & Ulijn, R. V. Biocatalytic self-assembly of supramolecular charge-transfer nanostructures based on n-type semiconductor-appended peptides. Angew. Chem. Int. Ed. 53, 5882–5887 (2014).
Trausel, F. et al. Catalysis of supramolecular hydrogelation. Acc. Chem. Res. 49, 1440–1447 (2016).
Yang, Z. et al. Enzymatic formation of supramolecular hydrogels. Adv. Mater. 16, 1440–1444 (2004).
Qin, X. et al. Enzyme-triggered hydrogelation via self-assembly of alternating peptides. Chem. Commun. 49, 4839–4841 (2013).
Pappas, C. G., Sasselli, I. R. & Ulijn, R. V. Biocatalytic pathway selection in transient tripeptide nanostructures. Angew. Chem. Int. Ed. 54, 8119–8123 (2015).
Pappas, C. G. et al. Dynamic peptide libraries for the discovery of supramolecular nanomaterials. Nat. Nanotechnol. 11, 960–967 (2016).
Ardoña, H. A. M. & Tovar, J. D. Peptide π-electron conjugates: organic electronics for biology? Bioconjug. Chem. 26, 2290–2302 (2015).
Gololobov, M. Y., Voyushina, T. L., Stepanov, V. M. & Adlercreutz, P. Nucleophile specificity in α-chymotrypsin- and subtilisin-(Bacillus subtilis strain 72) catalyzed reactions. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1160, 188–192 (1992).
Harada, N. & Nakanishi, K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 5, 257–263 (1972).
Kumar, M. et al. A dynamic supramolecular polymer with stimuli-responsive handedness for in situ probing of enzymatic ATP hydrolysis. Nat. Commun. 5, 5793-5800 (2014).
Gawroński, J., Brzostowska, M., Kacprzak, K., Kołbon, H. & Skowronek, P. Chirality of aromatic bis-imides from their circular dichroism spectra. Chirality 12, 263–268 (2000).
Wang, C., Wang, Z. & Zhang, X. Amphiphilic building blocks for self-assembly: from amphiphiles to supra-amphiphiles. Acc. Chem. Res. 45, 608–618 (2012).
Rahman, A. R. A., Justin, G. & Guiseppi-Elie, A. Bioactive hydrogel layers on microdisk electrode arrays: impedimetric characterization and equivalent circuit modeling. Electroanalysis 21, 1135–1144 (2009).
Sun, H. et al. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 9, 817–823 (2017).
Boekhoven, J. et al. Catalytic control over supramolecular gel formation. Nat. Chem. 5, 433–437 (2013).
Zhang, A. & Lieber, C. M. Nano-bioelectronics. Chem. Rev. 116, 215–257 (2016).
Marchesan, S., Ballerini, L. & Prato, M. Nanomaterials for stimulating nerve growth. Science 356, 1010–1011 (2017).
Acknowledgements
The authors acknowledge staff at the Biomolecular Spectroscopy Facility for Circular Dichroism, Imaging Suite Facility and Nanofabrication Facility, all of which are part of the Advanced Science Research Center at the Graduate Center, City University of New York. The research leading to these results received funding from the US Air Force (AFOSR, grants FA9550-15-1-0192 and FA9550-014-1-0350), US Army Research Laboratory and US Army Research Office under contract/grant number W911NF-16-1-0113.
Author information
Authors and Affiliations
Contributions
M.K. and R.V.U. conceived the idea, designed and discussed the concepts and experiments, and analysed the data. M.K. performed the experimental work. N.I. and A.H. designed and performed the electronic transport measurements and analysed the data. N.W. performed and analysed the infrared spectroscopy (IR) measurement. V.N. performed the atomic force microscopy experiment. M.K., R.V.U., N.I. and A.H. co-wrote the paper. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, and Supplementary Figures.
Rights and permissions
About this article
Cite this article
Kumar, M., Ing, N.L., Narang, V. et al. Amino-acid-encoded biocatalytic self-assembly enables the formation of transient conducting nanostructures. Nature Chem 10, 696–703 (2018). https://doi.org/10.1038/s41557-018-0047-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0047-2
This article is cited by
-
Biomimetic chiral hydrogen-bonded organic-inorganic frameworks
Nature Communications (2024)
-
Mechanosensitive non-equilibrium supramolecular polymerization in closed chemical systems
Nature Communications (2023)
-
Applications of synthetic biology in medical and pharmaceutical fields
Signal Transduction and Targeted Therapy (2023)
-
Programmable supramolecular chirality in non-equilibrium systems affording a multistate chiroptical switch
Nature Communications (2023)
-
Quick photofabrication of functional nanospheres from de novo designed peptides for NIR fluorescence and MR imaging
Nano Research (2023)