Abstract
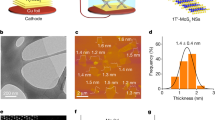
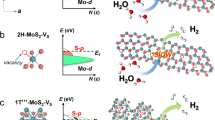
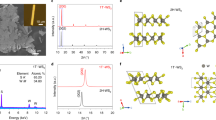
Phase control plays an important role in the precise synthesis of inorganic materials, as the phase structure has a profound influence on properties such as conductivity and chemical stability. Phase-controlled preparation has been challenging for the metallic-phase group-VI transition metal dichalcogenides (the transition metals are Mo and W, and the chalcogens are S, Se and Te), which show better performance in electrocatalysis than their semiconducting counterparts. Here, we report the large-scale preparation of micrometre-sized metallic-phase 1T′-MoX2 (X = S, Se)-layered bulk crystals in high purity. We reveal that 1T′-MoS2 crystals feature a distorted octahedral coordination structure and are convertible to 2H-MoS2 following thermal annealing or laser irradiation. Electrochemical measurements show that the basal plane of 1T′-MoS2 is much more active than that of 2H-MoS2 for the electrocatalytic hydrogen evolution reaction in an acidic medium.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sun, S. C., Murray, B., Weller, D., Folks, L. & Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287, 1989–1992 (2000).
Thelander, C., Caroff, P., Plissard, S., Dey, A. W. & Dick, K. A. Effects of crystal phase mixing on the electrical properties of InAs nanowires. Nano Lett. 11, 2424–2429 (2011).
Park, C. H., Cheong, B.-H., Lee, K.-H. & Chang, K. J. Structural and electronic properties of cubic, 2H, 4H, and 6H SiC. Phys. Rev. B 49, 4485–4493 (1994).
Kim, J., Lee, Y. & Sun, S. Structurally ordered FePt nanoparticles and their enhanced catalysis for oxygen reduction reaction. J. Am. Chem. Soc. 132, 4996–4997 (2010).
Fan, Z. et al. Synthesis of 4H/fcc noble multimetallic nanoribbons for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 138, 1414–1419 (2016).
Voiry, D., Mohite, A. & Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 44, 2702–2712 (2015).
Chhowalla, M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013).
Lukowski, M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013).
Acerce, M., Voiry, D. & Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotech. 10, 313–318 (2015).
Keum, D. H. et al. Bandgap opening in few-layered monoclinic MoTe2. Nat. Phys. 11, 482–486 (2015).
Py, M. A. & Haering, R. R. Structural destabilization induced by lithium intercalation in MoS2 and related compounds. Can. J. Phys. 61, 76–84 (1983).
Wypych, F. & Schollhorn, R. 1T-MoS2, a new metallic modification of molybdenum disulfide. J. Chem. Soc. Chem. Commun. 1386–1388 (1992).
Zeng, Z. et al. Single-layer semiconducting nanosheets: high-yield preparation and device fabrication. Angew. Chem. Int. Ed. 50, 11093–11097 (2011).
Lin, Y.-C., Dumcenco, D. O., Huang, Y.-S. & Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotech. 9, 391–396 (2014).
Kang, Y. M. et al. Plasmonic hot electron induced structural phase transition in a MoS2 monolayer. Adv. Mater. 26, 6467–6471 (2014).
Duerloo, K.-A. N., Li, Y. & Reed, E. J. Structural phase transitions in two-dimensional Mo- and W-dichalcogenide monolayers. Nat. Commun. 5, 4214 (2014).
Mahler, B., Hoepfner, V., Liao, K. & Ozin, G. A. Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136, 14121–14127 (2014).
Geng, X. et al. Pure and stable metallic phase molybdenum disulfide nanosheets for hydrogen evolution reaction. Nat. Commun. 7, 10672 (2016).
Chou, S. S. et al. Understanding catalysis in a multiphasic two-dimensional transition metal dichalcogenide. Nat. Commun. 6, 8311 (2015).
Yin, Y. et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 138, 7965–7972 (2016).
Chung, D. Y. et al. Edge-exposed MoS2 nano-assembled structures as efficient electrocatalysts for hydrogen evolution reaction. Nanoscale 6, 2131–2136 (2014).
Li, H. et al. From bulk to monolayer MoS2: evolution of Raman scattering. Adv. Funct. Mater. 22, 1385–1390 (2012).
Yang, D., Sandoval, S. J., Divigalpitiya, W. M. R., Irwin, J. C. & Frindt, R. F. Structure of single-molecular-layer MoS2. Phys. Rev. B 43, 12053–12056 (1991).
Calandra, M. Chemically exfoliated single-layer MoS2: stability, lattice dynamics, and catalytic adsorption from first principles. Phys. Rev. B 88, 245428 (2013).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2. Nano Lett. 10, 1271–1275 (2010).
Castellanos-Gomez, A. et al. Laser-thinning of MoS2: on demand generation of a single-layer semiconductor. Nano Lett. 12, 3187–3192 (2012).
Kappera, R. et al. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 13, 1128–1134 (2014).
Cho, S. et al. Phase patterning for ohmic homojunction contact in MoTe2. Science 349, 625–628 (2015).
Voiry, D. et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 15, 1003–1009 (2016).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48–54 (2016).
Acknowledgements
This work was supported by MOE under AcRF Tier 2 (ARC 19/15, nos. MOE2014-T2-2-093, MOE2015-T2-2-057 and MOE2016-T2-2-103) and AcRF Tier 1 (2016-T1-001-147 and 2016-T1-002-051), and NTU under a Start-Up Grant (M4081296.070.500000) in Singapore. It was also supported by the Joint Research Fund for Overseas Chinese, Hong Kong and Macao Scholars (grant no. 51528201). Q.X. acknowledges support from the Singapore National Research Foundation via an NRF Investigatorship Award (NRF-NRFI2015-03), Singapore MOE AcRF Tier2 grant (MOE2015-T2-1-047) and Tier1 grant (RG 113/16). Z.L. acknowledges support from the Singapore National Research Foundation under NRF RF award no. NRF-RF2013-08. The authors acknowledge the Facility for Analysis, Characterization, Testing and Simulation at Nanyang Technological University, Singapore, for use of their electron microscope (and/or X-ray) facilities.
Author information
Authors and Affiliations
Contributions
H.Z. proposed the research direction and guided the project. Y.Y., G.-H.N., Q.H. and X.-J.W. conceived the idea, designed the experiments and drafted the manuscript with H.Z., synthesized the materials, fabricated the devices, and analysed the data. K.Z. and Q.Z. helped with Raman and photoluminescence mapping measurements. X.W. and Q.X. conducted the temperature-dependent electrical property measurements. Z.Y. and L.G. carried out the STEM characterization. J.C., Q.M., M.Z. and Z.L. helped draft the manuscript. B.L. carried out XPS characterization. F.-R.R. and H.L. carried out AFM characterization. Y.D. analysed the XAFS results. Z.L., X.H. and W.H. helped revise the manuscript. All authors read the manuscript and agreed with its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary synthesis, characterization details, electrochemical measurements and analysis; Supplementary Figures 1–17 and Supplementary Tables 1 & 2
Supplementary Video
In situ Raman observation of the phase transformation process from 1T’-MoS2 to 2H-MoS2 under laser irradiation
Rights and permissions
About this article
Cite this article
Yu, Y., Nam, GH., He, Q. et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nature Chem 10, 638–643 (2018). https://doi.org/10.1038/s41557-018-0035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0035-6
This article is cited by
-
High intrinsic phase stability of ultrathin 2M WS2
Nature Communications (2024)
-
Phase-selective in-plane heteroepitaxial growth of H-phase CrSe2
Nature Communications (2024)
-
Optical and electrical anisotropy regulation engineering of low-dimensional materials toward polarized detection and imaging applications
Rare Metals (2024)
-
Two-dimensional Van der Waals heterostructures based chalcogenide for photovoltaic applications: a DFT study
Optical and Quantum Electronics (2024)
-
1T′-transition metal dichalcogenide monolayers stabilized on 4H-Au nanowires for ultrasensitive SERS detection
Nature Materials (2024)