Abstract
Nutrient availability is central for T-cell functions and immune responses. Here we report that CD8+ T-cell activation and anti-tumour responses are strongly potentiated by the non-essential amino acid Asn. Increased Asn levels enhance CD8+ T-cell activation and effector functions against tumour cells in vitro and in vivo. Conversely, restriction of dietary Asn, ASNase administration or inhibition of the Asn transporter SLC1A5 impairs the activity and responses of CD8+ T cells. Mechanistically, Asn does not directly alter cellular metabolic fluxes; it instead binds the SRC-family protein tyrosine kinase LCK and orchestrates LCK phosphorylation at Tyr 394 and 505, thereby leading to enhanced LCK activity and T-cell-receptor signalling. Thus, our findings reveal a critical and metabolism-independent role for Asn in the direct modulation of the adaptive immune response by controlling T-cell activation and efficacy, and further uncover that LCK is a natural Asn sensor signalling Asn sufficiency to T-cell functions.
Similar content being viewed by others
Main
T lymphocytes are key components of the adaptive immune system and protect the host from infection and cancer. T-cell survival and expansion following antigen stimulation and inflammatory signals require both extrinsic and intrinsic incentives; and metabolic reprogramming has recently emerged as a critical factor for determining T-cell fate and memory formation1,2,3. Metabolic switch to glycolytic flux and glutaminolysis triggered by T-cell antigen receptors (TCR) occurs early after activation to provide energy and building blocks for T-cell expansion and effector functions4,5,6. Furthermore, elevated mitochondrial capacity and fatty-acid oxidation are fundamental for memory CD8+ T-cell development7,8,9. Interestingly, the availability of certain nutrients, including Ser, kynurenine and l-Arg, in the micro-environment can impact T-cell survival, proliferation and effector responsivenesses10,11,12,13,14. Although the effect of metabolic alteration on T-cell expansion and survival becomes increasingly clear, the mechanisms underlying the metabolic manipulation of CD8+ T-cell activation remain poorly understood.
Here we report that Asn strongly enhances CD8+ T-cell activation both in vitro and in vivo. Dietary restriction of Asn or inhibition of Asn uptake impairs T-cell activation and differentiation into memory-like cells. Mechanistically, Asn can directly bind to LCK and induce its Tyr394 phosphorylation, consequently activating LCK signalling to promote T-cell activation and improve T-cell responses to pathogens or malignant cells.
Results
Asn promotes CD8+ T-cell activation
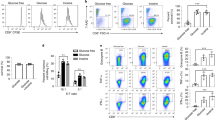
We sought to determine whether Asn influences immune functions by measuring its effect on CD8+ T-cell responses. Strikingly, Asn depletion resulted in a sharp reduction in CD8+ T-cell activation triggered by anti-CD3 and -CD28 antibodies (Fig. 1a). Conversely, the addition of Asn, possibly 100 µM at most, could sufficiently potentiate CD8+ T-cell activation (Fig. 1b and Extended Data Fig. 1a–d). Cellular Asn is equivalent to Asp, and asparagine synthetase (ASNS) catalyses the only route to generate Asn from Asp. However, unlike Asn, the activity of CD8+ T cells was unaffected by the depletion or addition of Asp (Fig. 1a,b); this was unlikely to be due to a defect in Asp uptake, as a significant accumulation of cellular 15N-Asp and reduction in media 15N-Asp were found when T cells were cultured with 15N-Asp during activation (Extended Data Fig. 1a). Thus, these data indicate a specific role for Asn in modulating CD8+ T-cell activation. Unsurprisingly, Asn itself could not trigger CD8+ T-cell activation when antigen was absent (Extended Data Fig. 1e–g, column 1 versus column 6).
a–c,e,f, Naive CD8+ T cells from mice were activated by anti-CD3 and -CD28 antibodies (unless otherwise indicated) in complete medium (Comp) and medium depleted (−) of either Asn or Asp for 24 h (to detect CD69 expression) or 40 h (to detect CD44 expression; a), or medium lacking Asn and Asp (Ctrl, control) and medium containing either Asn or Asp for 40 h (b,c,e,f), and analysed for expression of the indicated proteins or cytokines using FACS. d, Proliferation of mouse naive CD8+ T cells activated in Comp medium or medium depleted of the indicated amino acids for 4 d. e, Representative flow cytometry plots are shown with the percentage of cells in each quadrant indicated (right). In f, The cell culture medium was supplied with 10% dialyzed FBS. g–j, CD8+ T cells from mice were activated in Ctrl medium or medium containing Asn and/or ASNase (10 µg ml−1) for 40 h. The number (g) and size (h) of the cells, levels of intracellular and extracellular Asn (i) and surface expression of the indicated proteins (j) were analysed. k, CD25 expression and cytokine production by human CD8+ T cells (n = 3 independent human donors) activated in Ctrl medium or Asn medium with or without ASNase for 40 h. l–n, OT-I CD8+ T cells were cultured with B16-OVA melanoma cells in the absence or presence of Asn or ASNase for 40 h. The surface expression of CD25 and CD44 (l) and cytokine production (m) by OT-I CD8+ T cells as well as the percentage of annexin V+ B16-OVA cells (relative to total B16-OVA cells; n) were measured by FACS analysis. o,p, Naive CD8+ T cells from mice were cultured in Ctrl or EG-7 cell-conditioned medium (CM) with or without ASNase (10 μg ml−1) for 40 h. The surface expression of CD25 and CD44 on CD8+ T cells (o), and Asn (p, top) and Asp levels (p, bottom) in the media were measured. All data are the mean ± s.e.m.; n = 3 independent wells per experiment. Two-tailed Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant. Representative FACS plots for a–c,e,f,j–o (Supplementary Figs. 1 and 2) and numerical source data are provided.
Activated T cells transited from a naive state are able to proliferate rapidly and produce inflammatory cytokines15,16. Consistent with this, Asn supplementation resulted in an increased percentage of CD8+ T cells expressing interferon γ (IFN-γ), tumour necrosis factor α (TNF-α) and granzyme B (GzmB; Fig. 1c). A lack of l-Arg, Gln or Ser is known to limit T-cell proliferation12,13. Similar to these molecules, depletion of Asn noticeably reduced CD8+ T-cell expansion (Fig. 1d). This pro-proliferative effect of Asn was further verified by carboxyfluorescein diacetate succinimidyl ester (CFSE), Ki67 and BrdU staining (Extended Data Fig. 1h–j). Moreover, TCR-transgenic OT-I CD8+ T cells (specific for OVA257–264)17 that were supplied with Asn were markedly activated in response to OVA-peptide stimulation (Extended Data Fig. 1k,l).
Following antigen-specific and inflammatory signals, naive T cells soon undergo rapid division and subsequent differentiation15,18,19. Notably, Asn treatment resulted in a significant reduction in the naive CD8+ T-cell population and increased percentages of CD62LlowCD44highCD8+ and CD62LhighCD44highCD8+ T cells (Fig. 1e). Moreover, treatment with Asn, but not Asp, increased the population of CD8+ T cells with stemness properties, suggesting that Asn regulates the activation and differentiation of CD8+ T cells (Fig. 1f). Parenthetically, Asn-enhanced CD8+ T-cell activation was not reliant on serum components (Extended Data Fig. 1m–q).
In line with the above findings, Asn supplementation increased the overall number and size of the CD8+ T cells (Fig. 1g,h). Conversely, removal of Asn by treating cells with ASNase (Fig. 1i), an enzyme that hydrolyzes Asn into Asp and ammonia, reversed the effect of Asn on the CD8+ T cells (Fig. 1g,h,j). Similarly, treatment of human CD8+ T cells with Asn augmented the cell-activation capacity and increased the population of IFN-γ+CD8+ and TNF-α+CD8+ T cells; however, these effects were reduced when the cells were then supplied with ASNase (Fig. 1k and Extended Data Fig. 1r,s). These results were strengthened by the observations that ASNase had a minimal effect on the levels of Gln and Glu (Extended Data Fig. 2a–d), and Glu did not impact CD8+ T-cell activation (Extended Data Fig. 2e,f).
In addition, Asn enhanced human T lymphocyte Jurkat cell activation in a dose dependent manner (Extended Data Fig. 2g–j). Moreover, a noticeable increase in the activation of OT-I mouse CD8+ T cells stimulated by B16-OVA melanoma cells was observed when Asn was present (Fig. 1l,m). However, these effects were completely reversed following the addition of ASNase (Fig. 1l,m). Co-culture with activated CD8+ T cells triggered apoptosis of B16-OVA cells, and this phenomenon was more obvious in Asn-treated cells and largely suppressed by ASNase supplementation (Fig. 1n).
We next examined the cellular response to antigen stimulation following inhibition of de novo Asn synthesis. Expression of ASNS remarkably increased during activation but was almost undetectable in the naive state (Extended Data Fig. 3a,b). Intriguingly, the levels of cellular Asn declined during the early period of antigen stimulation, whereas both the extracellular and intracellular levels of Asn increased sharply at 48 h (Extended Data Fig. 3c). These findings suggest that Asn may be critical for the onset and/or maintenance of CD8+ T-cell activation and therefore heavily consumed during activation. In support of this idea, Asn did not affect the activated CD8+ T-cell state further (Extended Data Fig. 3d). Intriguingly, inhibition of ASNS by l-albizziine moderately decreased CD8+ T-cell activation (Extended Data Fig. 3e–h), indicating that, newly synthesised Asn has a lower impact on CD8+ T-cell activation compared with extracellular Asn.
The cytokine interleukin-2 (IL-2) promotes CD8+ T-cell activation and expansion, and increases the cytotoxicity of CD8+ T cells20. Following antigenic stimulation, Asn enhanced the survival and reduced apoptosis of CD8+ T cells in the presence of IL-2 (Extended Data Fig. 3i–k). In contrast, Asn failed to affect the survival, apoptosis and proliferation of activated CD8+ T cells that were cultured without IL-2 (Extended Data Fig. 3l–n), further supporting the idea that Asn regulates the transition of naive CD8+ T cells into the active state but not the functions of the activated cells.
Many tumour cells produce and secrete a large amount of Asn. Notably, naive CD8+ T cells cultured in medium from mouse EG-7 lymphoma cells and human lung cancer H1299 cells displayed an increased capacity for activation, and ASNase supplementation completely reversed this effect, thereby providing an argument for the involvement of Asn in this event (Fig. 1o,p and Extended Data Fig. 3o,p). Gln starvation resulted in a reduction in the levels of Asn in the culture media of tumour cells (Extended Data Fig. 3q). Consistent with this, the culture medium from glutamine-starved EG-7 cells had a markedly reduced ability to promote CD8+ T-cell activation (Extended Data Fig. 3r). Together, these data demonstrate that increased extracellular Asn levels enhance CD8+ T-cell activation. Notably, the finding of CD8+ T-cell sensing of tumour-cell-secreted Asn reveals a potential mechanism underlying the detection of de novo tumorigenesis by the immune system.
Asn potentiates CD8+ T-cell responses in vivo
We next investigated whether environmental Asn could influence T-cell responses in vivo. Mice maintained on a diet lacking Asn were subjected to intraperitoneal (i.p.) injection of Asn or PBS (control). Compared with the control animals, the Asn-treated mice displayed increased levels of blood Asn (Fig. 2a), spleen weights (Extended Data Fig. 4a) and numbers of cells in the spleen and lymph nodes (Extended Data Fig. 4b). However, the weight (Extended Data Fig. 4c) as well as the homeostatic status of the CD4+ and CD8+ T cells in the spleens and lymph nodes of the Asn-treated mice were not significantly changed (Extended Data Fig. 4d,e). Notably, we observed an increased population of CD25+CD8+ T cells as well as central memory-like CD8+ T cells in mice injected with Asn (Fig. 2b,c and Extended Data Fig. 4f,g). In addition, the Asn-fed mice had a relatively higher percentage of activated CD4+ T cells and memory-like CD4+ T cells (Extended Data Fig. 4h–j), suggesting that Asn not only affects the activity of CD8+ T cells but may also have a broad impact on T-cell functions.
a–c, C57BL/6 mice maintained on a diet lacking Asn and Asp were i.p. injected with PBS (Ctrl) or Asn every 2 d, for 30 d. The blood Asn levels (a), and percentage of CD25+CD8+ T cells in the spleen (b) and lymph nodes (c) were measured (Ctrl, n = 5; Asn, n = 6). d–f, Levels of blood Asn (d), and percentage of CD25+CD8+ T cells in the spleens (e) and lymph nodes (f) of mice on a normal diet, which were i.p. injected with PBS (Ctrl) or ASNase (0.2 mg per mouse) every 2 d, for 30 d (n = 6 or 7 mice per group). g, Schematic of the OT-I adoptive transfer and LmOVA infection experiment. OVA-specific (CD45.2+) CD8+ naive T cells were transferred into CD45.1+ host mice fed with control or Asn-supplemented water and the mice were then infected with LmOVA for another 7 d. h,i, The percentage and number of OVA-specific (CD45.2+) CD8+ T cells in the spleens (h) and lymph nodes (i) of the mice from g were measured (n = 5 mice per group). j, C57BL/6 mice that had been orally administered Asn-free (Ctrl) or -supplemented water were subjected to LmOVA infection and then rechallenged with a lethal dose of virulent LmOVA on the normal diet, as indicated. k,l, The percentage and number of OVA-TET+CD8+ T cells (k), and percentage of TNF-α+ and IFN-γ+ CD8+ T cells (l) in the spleens of the infected mice from j were measured 7 d after virulent LmOVA rechallenge (n = 5 mice per group). m,n, CD45.1+ mice on an Asn-free diet were i.p. injected with PBS (Ctrl) or Asn, adoptively transferred with OT-I CD45.2+CD8+ naive T cells and injected with B16-OVA cells, as illustrated in Extended Data Fig. 5j. The percentage and number of CD44+CD8+ T cells (m), and percentage of central (CD62LhighCD44high) and effector (CD62LlowCD44high) memory-like CD8+ T cells (n) in the spleens of the mice were analysed 16 d later (n = 5 mice per group). b,c,e,f,k–n, Cell percentages are expressed as a percentage of the total CD8+ T cells. Blood samples in a and d were 5 times diluted before assaying for Asn levels. h,i,k,l,m, Representative FACS plots are shown (h,i,k,l, left and m, right); the percentages of cells in each quadrant are indicated. All data are the mean ± s.e.m. Two-tailed Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant. Numerical source data for a–f,h,i,k–n and representative FACS plots (Supplementary Fig. 3) are provided.
In agreement with these findings (Fig. 2a–c and Extended Data Fig. 4a–j), Asn deprivation by ASNase in mice maintained on a diet containing Asn led to a marked decrease in the Asn levels in the blood (Fig. 2d), smaller spleens and reduced numbers of cells in the spleen and lymph nodes of the mice (Extended Data Fig. 4k–l). However, the overall percentage of CD4+ and CD8+ T cells was unchanged (Extended Data Fig. 4m). Notably, the mice that were injected with ASNase had reduced numbers of activated CD8+ (Fig. 2e,f), central memory-like CD8+ and, in a tissue-specific manner, effector memory-liked CD8+ T cells (Extended Data Fig. 4n,o). Similar findings were obtained for CD4+ T cells (Extended Data Fig. 4p–r). Consistent with this, culturing with serum from ASNase-administered mice significantly impeded CD8+ T-cell activation (Extended Data Fig. 4s).
We further examined T-cell responses to infection with OVA-expressing Listeria monocytogenes (LmOVA) in vivo (Fig. 2g). Notably, a dietary restriction of Asn reduced the number of OT-I CD8+CD45.2+ T cells in both the spleens and lymph nodes of congenic CD45.1 mice adoptively transferred with naive OT-I CD45.2+CD8+ T cells (Fig. 2h,i). We also examined whether Asn affects endogenous T-cell responses to Listeria infection (Extended Data Fig. 5a). Similarly, the weight of the spleens and the overall percentage of CD8+ and CD4+ T cells in the infected mice were unchanged (Extended Data Fig. 5b–g). However, the percentages of endogenous OT-I CD8+ T cells were reduced in mice on the Asn-free diet compared with the animals maintained on the control diet (Extended Data Fig. 5h,i).
Enhancement of CD8+ T-cell activation and the memory T-cell pool may potentiate the T-cell response and pathogen clearance following rechallenge12,21. We thus investigated whether availability of Asn during T-cell priming influences protective immunity (Fig. 2j). The analysis of the spleens of the mice that were initially fed with Asn revealed a significant elevation in the percentage and number of OT-I CD8+ T cells 30 d post rechallenge (Fig. 2k). Consistent with this, the mice that were administered Asn for the duration of the primary LmOVA response displayed increased IFN-γ+CD8+ and TNF-α+CD8+ T-cell populations, demonstrating that Asn can promote secondary T-cell responses to LmOVA (Fig. 2l).
Tumour-cell recognition by T cells induces effector and memory T-cell responses. Following malignancy, Asn-treated congenic CD45.1 mice adoptively transferred with naive OT-I CD45.2+CD8+ T cells had elevated numbers of OT-I CD8+CD45.2+ T cells (Fig. 2m and Extended Data Fig. 5j,k). Moreover, higher percentages of effector and central memory-like CD8+ T cells were found in the Asn-treated mice (Fig. 2n and Extended Data Fig. 5l). Collectively, these findings suggest that Asn influences the activity and responses of CD8+ T cells in vivo.
Cellular uptake, not the exchange factor role, is required for Asn to promote CD8+ T-cell activation
Asn is an amino-acid exchange factor in proliferating cells22. Similarly, Asn starvation significantly lowered Ser uptake in CD8+ T cells, despite the fact that Asn uptake was unaffected by the depletion of Ser and Gly (Ser + Gly; Fig. 3a,b). However, Ser + Gly deficiency failed to influence Asn-enhanced CD8+ T-cell activation (Fig. 3c) and Asn supplementation was still able to improve CD8+ cell activation even when the cells were cultured in amino-acid-free medium (Fig. 3d), suggesting that the role of Asn as an amino-acid exchange factor is not required for its promoting effect on CD8+ T-cell activation.
a, Extracellular (left) and intracellular (right) quantities of 15N-Asn of naive CD8+ T cells from mice activated in complete medium (+) or medium depleted (−) of Ser and Gly in the presence of 0.5 mM 15N-Asn for 24 h (n = 3 independent wells). b, Extracellular (left) and intracellular (right) quantities of 13C-Ser of naive CD8+ T cells from mice cultured for 24 h in 0.5 mM 13C-Ser-supplemented medium containing no (−) or 0.4 mM Asn (n = 3 independent wells). a,b, Values were calculated as the peak area × 107 for 3 × 106 cells. c,d, Surface expression of CD69, CD25 and CD44 on CD8+ T cells stimulated in medium lacking or supplemented with Ser and Gly (c), or in amino-acid-free medium containing no or 0.2 mM Asn (d), as indicated (n = 3 independent wells). e, Relative mRNA expression of potential transporters for Asn uptake in tissues from human spleens (n = 4) and lymph nodes (n = 5). RPKM, reads per kilobase of transcript per million mapped reads. f,g, Jurkat cells treated with control siRNA (siCtrl) or siRNA against SLC1A5 (siSLC1A5) or SLC38A2 (siSLC38A2) were cultured in medium containing 15N-Asn for 2 h. The expression of SLC1A5 and SLC38A2 mRNA (f; determined using quantitative PCR with reverse transcription), and the cellular levels of 15N-Asn, Gln and Glu (g) were measured (n = 3 independent wells). Data are the normalized peak area × 104 for 15N-Asn and normalized peak area × 105 for Gln and Glu. h, Levels of 15N-Asn, Gln and Tyr in naive CD8+ T cells from mice (left) and Jurkat cells activated in medium containing 0.5 mM 15N-Asn for 2 h in the presence of increasing concentrations of V9302; a.u., arbitrary units. i, Mouse CD8+ T cells activated (3.5 μg ml−1 anti-CD3 and 1 μg ml−1 anti-CD28) in Asn or Asn-free medium in the presence or absence of V9302 for 12 h. The surface expression of CD25 and CD69 relative to the total CD8+ T cell populations was quantified (n = 3 independent wells). Representative flow cytometry plots are shown and the percentages of cells in the boxed areas are indicated (right). All data are the mean ± s.e.m. Two-tailed Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant. Representative FACS plots for c,d,i (Supplementary Fig. 4) and numerical source data for a–i are provided.
We then investigated whether cellular uptake is essential for the Asn-promoted activation of CD8+ T cells. As it remains unclear which transporters are involved in Asn import and exchange, we analysed the expression of several potential Asn transporters22 in human lymph-node and spleen tissues. Notably, high expression levels of the transporters SLC1A5 and SLC38A2 as well as three antiporters (SLC7A1, SLC7A5 and SLC1A4) potentially involved in Asn exchange function were found in these immune organs (Fig. 3e). Similar findings were also obtained in Jurkat cells (Extended Data Fig. 5m). As examined above, Asn augmentation of T-cell activation does not rely on its exchange function; we thus focused on the transporters SLC1A5 and SLC38A2. Activation of CD8+ T cells increased the expression of SLC1A5, but not SLC38A2, to some extent (Extended Data Fig. 5n,o). Consistent with this, ablation of SLC1A5, but not SLC38A2, reduced the Asn uptake (Fig. 3f,g). Interestingly, the cellular levels of Gln, but not Glu, were significantly reduced following SLC1A5 silencing (Fig. 3f,g). Similarly, pharmacological inhibition of SLC1A5 dramatically reduced the levels of intracellular 15N-Asn and Gln but not Tyr (Fig. 3h). Thus, both Asn and Gln can use SLC1A5 for their importation into cells. Next, we examined the effect of Asn uptake on CD8+ T-cell activation by inhibiting SLC1A5 in the absence of Gln. SLC1A5 inhibition was sufficient to block the Asn-mediated enhancement of CD8+ T-cell activation (Fig. 3i and Extended Data Fig. 5p). Collectively, these findings suggest that Asn uptake mediated by SLC1A5 is critical for Asn-promoted CD8+ T-cell activation.
Asn-enhanced CD8+ T-cell activation is independent of mTORC and Gln
Next, we explored the mechanism(s) underlying T-cell activation by Asn. Upregulation of mTORC1 activity metabolically supports T-cell activation23, and Asn is able to stimulate mTORC1 activity indirectly22,24. Activation of CD8+ T cells indeed displayed increased mTORC1 activity, and the effect was strongly exacerbated by Asn supplementation (Extended Data Fig. 6a,b). However, although the mTORC1 inhibitor rapamycin could reduce T-cell activation, we still observed a substantial elevation of CD8+ T-cell activation induced by Asn (Extended Data Fig. 6c), indicating that upregulation of mTORC1 activity may contribute to, but is not sufficient for, Asn-enhanced CD8+ T-cell activation.
When Gln is limited, Asn is used for protein synthesis in many tumour cells25. Similarly, the addition of Asn during the activation of T cells, promoted protein synthesis and significantly restored messenger RNA translation when Gln was absent or scarce (Extended Data Fig. 6d). Moreover, Gln limitation reduced the proteasome inhibition-induced accumulation of short-lived proteins (MYC and NRF2), and Asn supplementation resulted in a noticeable restoration of this accumulation (Extended Data Fig. 6e). Thus, these data suggest a role for Asn in restoring translation in the absence of Gln. In keeping with this, Gln depletion or scarcity did not sufficiently block the enhancement of T-cell activation mediated by Asn (Extended Data Fig. 6f).
LCK signalling mediates Asn-promoted CD8+ T-cell activation and responses
T-cell activation is accompanied by metabolic reprogramming13 (Extended Data Fig. 7a,b). Although Asn supplementation changed the cellular levels of many metabolites (Extended Data Fig. 7a,b), isotope tracing using 15N-Asn demonstrated that the integrated Asn was not metabolized further (Fig. 4a,b), suggesting that Asn modulation of T-cell activation may not be attributable to its direct metabolic participation despite the fact that it can impinge on activation-induced T-cell metabolic reprograming.
a,b, Naive CD8+ T cells from mice were activated in medium containing 400 µM 15N-Asn for 0, 6 or 12 h, as indicated. a, The cellular abundances of metabolites labelled with the stable isotope 15N for the indicated time periods were analysed by liquid chromatography with tandem mass spectrometry (LC–MS/MS). The heat map shows the differences in metabolite abundances. b, Mass isotopomer distribution (MID) of cellular amino acids at 12 h. The incorporation of 15N atoms from Asn are denoted as M + n, where n is the number of 15N atoms, M means mass isotopomer. c, Mouse CD8+ T cells left unstimulated (naive) or stimulated (10 µg ml−1 anti-CD3 and 1 µg ml−1 anti-CD28 antibodies) for 12 min in the presence of increasing quantities of Asn were analysed by western blotting. d, Mouse CD8+ T cells in medium lacking Asn and Asp (vehicle) or Asn medium were stimulated for the indicated times and protein expression was analysed by western blotting. e, CD45.1 C57BL/6 mice on an Asn-free diet and treated with PBS (Ctrl) or Asn were adoptively transferred with naive OT-I CD45.2+CD8+ T cells and i.p. injected with 50 µg OVA peptide in complete Freund’s adjuvant (CFA) as indicated in Extended Data Fig. 7d. The expression levels of LCK phosphorylation in spleen OT-I CD8+ T cells at 12 h post treatment with the OVA peptide were analysed by FACS (relative to the total CD8+ cell population; n = 5 mice per group). f, Immunoblot analysis of naive CD8+ T cells from mice activated in medium containing Asn and/or ASNase for the indicated time periods. g,h, Confocal images of phosphorylated (p-) LCK, ZAP70 and PI3K (g), as well as AKT and LAT171 (h) clustering in naive CD8+ T cells stimulated with or without Asn for 10 min (left). The total fluorescence intensity (tFI) was quantified (right; n = 48 cells). Scale bars, 4 µm. All data are the mean ± s.e.m. Two-tailed Student’s t-test; *P < 0.05, ***P < 0.001 and ****P < 0.0001. Western blot data are representative of at least three independent experiments. Uncropped blots for c,d,f, representative FACS plots for e (Supplementary Fig. 5) and numerical source data for a,b,e,g,h are provided.
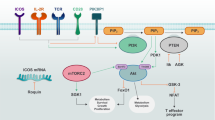
T-cell activation requires the Src family tyrosine kinase LCK to initiate antigen-specific TCR signalling26 (Extended Data Fig. 7c). Notably, when CD8+ T cells were supplied with Asn, we observed a remarkable enhancement in the phosphorylation of LCK (Y394) and the LCK substrates ZAP70 and PI3K during activation (Fig. 4c,d). Similar findings were also observed in vivo. Following OVA-peptide treatment, adoptively transferred CD45.1 mice displayed significantly elevated LCK phosphorylation in spleen OT-I CD8+ T cells when Asn was provided (Fig. 4e and Extended Data Fig. 7d). Conversely, treatment with ASNase reduced the phosphorylation of LCK, ZAP70 and PI3K (Fig. 4f). Moreover, using high-resolution imaging, we found elevated probable microclusters of phosphorylated TCR signalling proteins in Asn-treated CD8+ T cells (Fig. 4g,h). Together, these findings suggest a previously unappreciated role for Asn in the regulation of TCR signalling.
Addition of the LCK inhibitor PP2 totally blocked LCK and PI3K phosphorylation triggered by Asn. The PI3K inhibitor GDC-0032 affected LCK phosphorylation slightly but reduced PI3K phosphorylation in cells cultured with Asn (Fig. 5a and Extended Data Fig. 7e). In keeping with these findings, although the Asn feed enhanced CD8+ T-cell activation, supplementation with PP2 and GDC-0032 remarkably reduced (PP2 caused a total reversal) Asn-promoted T-cell activation (Fig. 5b,c). Consistent with this, the introduction of the mutant LCK(Y394F) reduced T-lymphocyte activation and blunted the susceptibility of these cells to Asn availability (Fig. 5d). In addition, OT-I adoptively transferred mice injected with Asn had an increased population of activated CD8+ T cells as well as effector and central memory-like CD8+ T cells (Fig. 5e–g,i–k and Extended Data Fig. 7f). Apparently, the administration of PP2 or GDC-0032 reduced T-cell activation and the number of spleen OT-I+ CD8+ T cells, thereby diminishing the difference between the control and Asn-treated mice (Fig. 5e–k). We further confirmed these findings using LmOVA infections (Extended Data Fig. 7g). The administration of Asn increased the population of activated, effector and central memory-like T cells, and treatment with PP2 totally reversed these effects (Fig. 5l–o). Together, these findings suggest that Asn enhances T-cell activation through LCK.
a, Immunoblot of the LCK, p-LCK, PI3K and p-PI3K proteins in mouse CD8+ T cells treated with Asn and/or PP2 or GDC-0032, as indicated, during stimulation. Data are representative of three independent experiments. b,c, Naive CD8+ T cells from mice were treated as in a. The percentage of CD25+CD8+ T cells and CD44+CD8+ T cells relative to total CD8+ T cells (b), and surface expression of PDL-1 and CTLA-4 on the CD8+ T cells (c) were measured (n = 3). d, Jurkat T cells expressing yellow fluorescent protein (YFP)–LCK or the YFP–LCK(Y394F) mutant were stimulated in control medium lacking Asn and Asp (Ctrl) or medium containing Asn. The percentage of CD25+CD8+ T cells relative to total CD8+ T cells was quantified (left; n = 3 independent experiments). Representative FACS plots are shown (right). e–k, CD45.1 C57BL/6 mice on an Asn-free diet and i.p. injected with Asn or PBS (−), or with/without PP2 (30 µg per mouse) or GDC-0032 (100 µg per mouse) were adoptively transferred with naive OT-I CD45.2+CD8+ T cells and injected with OVA peptide as indicated in Extended Data Fig. 7f. The percentage of CD44+CD8+ (e,i), CD62LhighCD44highCD8+ (f,j) and CD62LlowCD44highCD8+ (g,k) T cells relative to the total CD8+ T-cell population in the spleens (e–h) and lymph nodes (i–k) of the mice were measured. The overall percentage and number of OT-I CD8+ T cells in spleen were also determined (h, right; n = 5 mice per group). l–o, CD45.1 C57BL/6 mice on an Asn-free diet and i.p. injected with Asn and/or PP2 were adoptively transferred with naive OT-I CD45.2+CD8+ T cells and infected with LmOVA as indicated in Extended Data Fig. 7g. The percentage of CD44+CD8+ (l), CD62LhighCD44highCD8+ (m) and CD62LlowCD44highCD8+ (n) T cells (as a percentage of the total CD8+ T-cell population), and production of cytokines by CD8+ T cells (o) in the spleens of the mice were measured at day 11 (n = 5 mice per group). All data are the mean ± s.e.m. Two-tailed Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant. Uncropped blots for a, representative FACS plots for b–o (Supplementary Figs. 6,7) and numerical source data for b–o are provided.
Asn binds to LCK directly and promotes its activity
Whereas Tyr505 phosphorylation is inactivated, Tyr394 autophosphorylation activates LCK26. Interestingly, treatment with Asn dose-dependently increased Tyr394 phosphorylation and decreased Tyr505 phosphorylation (Fig. 6a,b), and directly activated LCK in vitro (Fig. 6c and Extended Data Fig. 8a). However, these effects were not evident following Asp treatment (Fig. 6a,b). We next performed an equilibrium binding assay using radioactive amino acids to investigate the possibility of Asn–LCK binding27. Purified recombinant LCK protein immobilized on beads was incubated with 3H-Asn, followed by washing and quantification. Notably, LCK bound to 3H-Asn in a dose-dependent manner, which could be fully competed by non-radiolabelled Asn (Fig. 6d). Specifically, 3H-Asn only bound to LCK; it did not bind the ZAP70 and SLP76 proteins (Fig. 6e). In contrast, Asp exhibited no binding affinity towards LCK and the other proteins (Fig. 6e). These findings were further confirmed using surface plasmon resonance (BIAcore; Fig. 6f); Asn also bound to LCK mutants (Y394F and Y505F; Extended Data Fig. 8b,c).
a, In vitro kinase assays of recombinant LCK proteins left untreated (Ctrl) or incubated with ATP and increasing concentrations of Asn or Asp for 15 min. LCK phosphorylation was analysed by western blotting (a) and enzyme-linked immunosorbent assay (ELISA) using anti-p-LCK (Y394) antibody (b). The results show the average values of the optical density at 450 nm (OD450) from triplicates. c, The activity of recombinant LCK proteins left untreated (Ctrl) or treated with either Asn or Asp for the indicated times in the presence of ATP was investigated by ELISA using biotin-poly (Glu4: Tyr, 4:1) as described in Methods (n = 3 biological replicates). d,e, 3H-radiolabelled Asn bound to purified recombinant LCK, but not ZAP70 and SLP76 (n = 3 biological replicates). Unlabelled amino acid was added where indicated (see Methods for details). e, 3H-radiolabelled Asp did not bind LCK, ZAP70 or SLP76. f, BIAcore measurements of the interaction between purified LCK and Asn or Asp. Graphs of equilibrium response units and compound concentrations are shown (middle and right). g,h, Absolute concentrations of Asn in mouse CD8+ T cells left unstimulated (naive) or stimulated in medium containing 0 or 400 µM Asn and/or 10 µg ml−1 ASNase for the indicated times. The Kd value of LCK for Asn is indicated by the dashed line (n = 3 independent wells). i,j, BIAcore measurements of the interaction between Asn and the purified LCK kinase domain (amino acids 231–509; i) and SH3-SH2 domain (amino acids 60–240; j). Graphs of the equilibrium response units and compound concentrations are shown (right). b–e,g,h, Statistical significance was determined using a two-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test (b,c) or a two-tailed Student’s t-test (d,e,g,h); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. d–f,i,j, Purified proteins were analysed by SDS–PAGE, followed by Coomassie blue staining (left). a,f,i,j, Data are representative of at least three independent experiments. All data are the mean ± s.e.m. MW, molecular weight markers. Uncropped blots for a and numerical source data for b–j are provided.
In addition, we assessed the binding of LCK to other amino acids. Unlike Asn, other amino acids did not bind to LCK, with the exception of Gly and His, which interacted weakly with LCK (Extended Data Fig. 8d–u). Neither Asn nor Asp bound to ZAP70, as measured using BIAcore (Extended Data Fig. 8v). Consistent with the in vitro BIAcore and radioactive-labelling data, cellular thermal shift and drug affinity responsive targets stability assays revealed direct binding between LCK and Asn inside cells (Extended Data Fig. 8w,x). Because Asn depletion reduced the LCK activity and T-cell activation, the cellular Asn concentrations should be sufficient for Asn binding to LCK. The Asn concentrations in both naive and activated cells were indeed higher than the dissociation constant (Kd) of LCK for Asn, making it probable that Asn is a physiologically relevant modulator of LCK activity (Fig. 6g). In keeping with this, when the cells were supplied with Asn elevated the cellular Asn abundances quickly and substantially resulting in increased LCK activity and T-cell functions (Fig. 6g). Conversely, the Asn concentrations in CD8+ T cells decreased following ASNase treatment, with the Kd decreasing from a value above that of LCK for Asn to below this value (Fig. 6h).
Phosphorylation controls LCK activity26. Blocking phosphorylation on Tyr394 (Y394F) largely reduced LCK activity, whereas inhibition of Tyr505 phosphorylation (Y505F) stimulated LCK activity (Extended Data Fig. 8y,z). Asn addition failed to significantly activate LCK(Y394F) and LCK(Y505F) (Extended Data Fig. 8y), and the Y394F mutation did not affect the Asn-mediated reduction of Tyr505 phosphorylation, whereas the Y505F mutation boosted Tyr394 phosphorylation and minimized the effect of Asn (Extended Data Fig. 8z). Thus, Asn might inhibit Tyr505 phosphorylation to promote Tyr394 autophosphorylation. To identify the region of binding, we compared the ability of various LCK deletion mutants to bind Asn and found that the binding site for Asn is the region between amino acids 240 and 320 on LCK within the kinase domain (Fig. 6I,j and Extended Data Fig. 9a–c).
Asn enhances the anti-tumour T-cell response
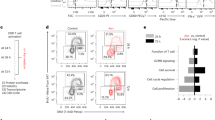
Next we investigate whether Asn potentiates anti-tumour T-cell responses. Naive OT-I CD8+ T cells pre-stimulated by the OVA257–264 peptide in control or Asn-supplemented medium were co-cultured with EG-7 or B16-OVA cells. Notably, Asn endowed OT-I CD8+ T cells with a higher cytotoxicity and capacity to kill tumour cells (Fig. 7a and Extended Data Fig. 9d). Furthermore, following intravenous injection of B16 cells, the Asn-treated mice had an increased percentage of activated CD8+ T cells, and effector and central memory-like T cells (Extended Data Fig. 9e–j). Conversely, ASNase treatment reduced CD8+ T-cell activation, the memory-like T-cell population and cytokine production (Extended Data Fig. 9k–p).
a, OVA-specific OT-I CD8+ T cells pre-activated in medium lacking both Asn and Asp (−) or with Asn (+) were cultured with EG-7 cells for 18 h. The relative cytotoxicity (left) and percentage of apoptotic CD8+ T cells (right) were determined (n = 3 independent wells). b,c, C57BL/6 mice were intravenously injected with B16-OVA cells, immunodepleted by whole-body irradiation13,33 and injected with naive OT-I CD8+ T cells pre-activated in medium lacking Asn and Asp (Ctrl) or Asn-supplemented medium (Asn), as illustrated in Extended Data Fig. 9q. The tumour multiplicity on day 17 (n = 7 mice per group; b, right) and mouse survival (n = 10 mice per group; c) were measured. Representative images of lung tumour multiplicity are shown (b, left). d, C57BL/6 mice were tail-vein injected with 4 × 105 B16-OVA cells and immunodepleted by irradiation twice (2 Gy each time). After 12 h, the mice were tail-vein injected with 8 × 105 CD8+ T cells pre-activated in the presence (Ctrl) or absence (−) of Arg and Asn. The tumour multiplicity in the lungs of the mice was measured on day 12 (n = 5; right) and the representative images of lungs are given. e–g, C57BL/6 mice on an Asn-free diet and intravenously injected with PBS (Ctrl) or Asn were subcutaneously (s.c.) administered B16-OVA cells as indicated (e), and the tumour burden (f) and survival (g) over time were assessed (n = 8 mice per group). h–j, C57BL/6 mice were subjected to B16-OVA-cell injection, whole-body irradiation, naive OT-I CD8+ T-cell transfer and treatment of with Asn or PBS (Ctrl), as illustrated in Extended Data Fig. 9s. h,i, The tumour burden (h) and survival (i) over time were assessed. Tumour-infiltrating CD8+ T-cell proliferation was determined by Ki67 staining (n = 8 mice per group). j, Percentage of CD44+CD8+ T cells and production of cytokines by CD8+ T cells in tumours relative to the total CD8+ cell population (n = 10 mice per group). a,b,d,f,h,j, Statistical significance was determined using a two-tailed Student’s t-test (a,b,d,j), or a two-way ANOVA, followed by Tukey’s multiple comparisons test (f,h); **P < 0.01, ***P < 0.001 and ****P < 0.0001. All data are the mean ± s.e.m. Representative FACS plots for a,j (Supplementary Fig. 8) and numerical source data for a–d,f–j are provided.
Remarkably, when adoptively transferred into immunodepleted mice injected with B16-OVA cells, Asn-treated OT-I CD8+ T cells exhibited a superior anti-tumour efficacy, as measured by the reduced tumour-cell metastasis and increased survival of the mice (Fig. 7b,c and Extended Data Fig. 9q). Conversely, Asn starvation endowed OT-I CD8+ T cells with reduced anti-tumour activity (Fig. 7d). Similarly, Asn rendered OT-I T cells with an increased capacity to suppress the growth of subcutaneous xenografted tumours (Extended Data Fig. 9r). To assess the effect of Asn on the endogenous anti-tumour immune response, we subcutaneously injected B16-OVA cells into mice pretreated with PBS or Asn. The mice that were treated with Asn displayed strong anti-tumour immune responses and increased survival (Fig. 7e–g). Moreover, naive OT-I CD8+ T cells primed by OVA in immunodepleted and tumour-bearing mice that were intraperitoneally injected with Asn also mounted an effective anti-tumour response (Fig. 7h,l and Extended Data Fig. 9s). Specifically, Asn endowed these tumour-infiltrated T cells with a higher capacity for activation, cytokine generation and even proliferation (Fig. 7j). Collectively, these data demonstrate that increased Asn levels enhance the activation of CD8+ T cells and their anti-tumour activity in vivo.
Discussion
The availability of specific nutrient(s) dramatically influences innate and/or adaptive immunity through manipulation of the antigen-specific T-cell expansion capacity and efficacy28. However, it remains unclear whether T-cell activation requires the direct involvement of certain nutrient(s), and in this work we sought to determine the role of Asn in T-cell differentiation29. We found that Asn can strongly enhance T-cell activation and the memory-like potential of T cells, demonstrating the importance of Asn as a crucial metabolic factor that can cause a sharp increase in T-cell immunity. More importantly, Asn serves as a signalling molecule in CD8+ T cells, which can be directly sensed by LCK.
Tumour cells outcompete T cells for nutrients (for example, glucose), leading to impeded T-cell effector function29. Similarly, T and tumour cells may also compete for Asn in the tumour micro-environment for proliferation and survival, particularly when Asn is scarce or tumour cells are defective in the de novo synthesis of Asn. However, many tumour cells usually have a high biogenetic activity that can enable them to produce and release Asn25,30,31 (Fig. 1p and Extended Data Fig. 3p), which can fuel the bioenergetic demands of both T and tumour cells for growth or preserve the anti-tumour potential of T cells. In this situation, tumour cells may be able to manipulate T-cell responses by changing the levels of Asn in the micro-environment. Given that Asn also positively regulates breast tumour-cell metastasis32, increasing the levels of Asn in the tumour micro-environment may have a protective role in preventing tumorigenesis by enhancing the T-cell responses but would also benefit tumour cells if they have escaped the immunosurveillance system. Under this condition, it is possible that Asn contributes to tumour-cell-mediated T-cell exhaustion in the tumour micro-environment. Nevertheless, the availability of Asn in vivo may exhibit complex and time-dependent functions in cancer immunosurveillance but would have pathophysiological and therapeutic implications for immunotherapy.
Methods
Antibodies and reagents
The antibodies that were used in this work are listed in Supplementary Table 1. The following reagents were purchased from the indicated sources: mouse IL-2 (R&D, 402-ML), EasySep mouse biotin selection kit (Stem Cell, 18556), CD8+ T isolation kit (Stem Cell, 19853), l-Gln (Corning, 25-005-CIR), l-Asn (ProSpec, ENZ-287), l-Albizziine (Goldbio, A-230-250), CFSE (Invitrogen, C34554), Percoll (GE Healthcare, 17089101), Ficoll (GE Healthcare, 17144002), Taselisib (GDC-0032; Selleck, 1282512-48-4), PP2 (Selleck, 172889-27-9), poly(Glu4-Tyr) peptide biotin conjugate (Millipore, 12-440). Brain heart infusion medium (Solarbio, A0360) and mouse special food (Asn-and-Asp-free) were purchased from Changzhou Shuyishuer Bio-tech Co., Ltd. The following reagents were purchased from Sigma: l-Asn monohydrate (A8381), l-Asp (A9256), l-Ser (S4311), l-Thr (T8441), l-Trp (T8941), l-Pro (P5607), l-Arg (A8094), l-Tyr disodium salt hydrate (T1145), l-Ala (A7469), l-Cys (C7352), l-His HCl (H5659), l-Ile (I7403), l-Leu (L8912), l-Lys HCl (L8662), l-Met (M5308), l-Phe (P5482), l-Val (V4638), N-acetyl-l-Glu (855642), Gly (V900144), l-Asn-(amide-15N) monohydrate (485896), anti-FLAG M2 affinity gel (A2220), FLAG peptide (F3290), collagenase IV (C5138) and Freund’s complete Adjuvant (F5581).
Semi-quantitative PCR with reverse transcription
Total RNA was isolated using a total RNA purification kit (GeneMark, TR01). The RNA (2 µg) was reverse transcribed to complementary DNA using the First-strand cDNA synthesis system (Thermo Scientific, K1621). For each sample, 0.2 µg cDNA product was used as a template to conduct quantitative PCR. The primer pairs that were used are listed in Supplementary Table 2. Quantitative PCR was performed using SYBR Green PCR master mix (GenStar, A301-10) on a CFX96 real-time PCR system (Bio-Rad). Gene expression of the gene of interest was normalized to that of β-actin.
Animals and human samples
Six- to eight-week-old C57BL/6 mice were purchased from Vital River Laboratory Animal Technology for CD8+ T-cell isolation. The mice were maintained under a 12/12 h light/dark cycle at 22–26 °C and were fed with sterile pellet food and water ad libitum. The animal facilities used in this study have been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and Institutional Animal Care and Use Committee (IACUC) of Tsinghua University. All of the mice were maintained in pathogen-free facilities and used strictly in accordance with the protocols approved by the IACUC of Tsinghua University. All of the animals used were matched for age and sex and randomly allocated to experimental groups. The study is compliant with all of the relevant ethical regulations regarding animal research and human participants. Human peripheral blood mononuclear cells were obtained from healthy volunteers under approved protocols of the Peking Union Medical College Hospital Ethics Board and we obtained informed consent from all of the participants. Relevant information on the human research participants is listed in Supplementary Table 3.
LCK kinase activity assays by ELISA
To measure the phosphorylation of Tyr394 on LCK, recombinant LCK proteins pre-incubated with a buffer containing 60 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM MnCl2, 3 µM NaVO4, 1 mM dithiothreitol and with or without 5 µg ml−1 Asn were incubated at 37 °C for 15 min. ATP was then added to the mixtures to a final concentration of 500 µM and incubated for 15 min at 37 °C. The reaction mixtures were boiled for 10 min at 98 °C and transferred into a 96-well ELISA plate (100 µl per well) to detect the phosphorylation of Tyr394 on LCK. For the ELISA, a 96-well ELISA plate with the mixtures was incubated at 37 °C for 2 h before the removal of the supernatant; the plate was then blocked with 100 µl 5% BSA for 1 h at 37 °C. After three washes in PBST buffer, 100 µl mouse anti-LCK p-Tyr394 antibody (1:2,000) was added and incubated for another 1 h at 37 °C. Next, the antibody was washed out with 200 µl PBST, goat anti-mouse IgG-HRP was added (100 µl per well) and incubated for 1 h before the addition of TMB buffer and subsequently TMB stop buffer according to manufacturer’s manual (BioLegend, 421101). The absorbance at 450 nm was measured using a microplate reader (Molecular Devices, SpectraMAX M2e).
For the determination of LCK activity by substrate phosphorylation of biotin-poly (Glu4: Tyr, 4:1), LCK proteins (1 µg ml−1) were incubated with 5 µg ml−1 poly (Glu4: Tyr, 4:1)-biotin in a buffer containing 60 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM MnCl2, 3 µM NaVO4 and 1 mM dithiothreitol in the presence of 100 µM Asp, 100 µM Asn or vehicle control. After an incubation of 15 min at 37 °C, ATP was added and incubated for another 15 min, followed by boiling at 98 °C. The ELISA plate was then loaded with 50 µg ml−1 streptavidin, blocked with 5% BSA and washed three times with PBST. The LCK reaction mixtures were then transferred into the plate wells (100 µl per well) and incubated for 15 min. Mouse anti-phospho-Tyr antibody 4G10 (100 µl; 1:5,000) was then added to the wells for a further incubation at 37 °C, followed by washing with PBST and incubation with 100 µl goat anti-mouse IgG-HRP for another 1 h. The LCK activity was measured and calculated (the absorbance at 450 nm) using a microplate reader.
Cell lines and culture conditions
B16-F10 and 293T cells were purchased from the American Type Culture Collection. The B16-OVA cells were a gift from the C. Xu laboratory (CAS, Shanghai). H1299 cells and EG-7 cells were provided by Y. Wang (Tsinghua University, Beijing) and Z. Dong (Tsinghua University, Beijing), respectively. CD8+ T cells were cultured in DMEM medium (Macgene, CM15022) supplemented with the same type and concentration of amino acids as RMPI-1640 (Macgene, CM10041), 10% FBS and/or Asn (100 µM, unless otherwise indicated). Specific assays used 10% dialysed FBS-supplemented media containing the indicated concentrations of amino acids. Moreover, T-cell special culture medium was supplemented with 50 mg ml−1 streptomycin, 50 U ml−1 penicillin and, unless otherwise indicated, 10 ng ml−1 IL-2. CD8+ T cells were stimulated with anti-CD3 (3.5 µg ml−1) and anti-CD28 (1 µg ml−1) antibodies for 40 h. H1299 and EG-7 cells were cultured in RPMI-1640 medium (Macgene, CM10041) supplemented with 10% FBS. B16-F10 and B16-OVA cells were cultured in DMEM medium (Gibco, C11995) supplemented with 10% FBS. All of the cell lines that were used have been authenticated and were tested for mycoplasma contamination.
Isolation and stimulation of T cells
Mouse T cells were isolated from the spleens and lymph nodes of the mice using a biotin selection kit (Stem Cell). Human T cells were isolated from peripheral blood mononuclear cells by Ficoll gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare). The CD8+ T cells were, unless otherwise indicated, stimulated with 3.5 µg ml−1 anti-CD3 and 1 µg ml−1 anti-CD28 antibodies for 40 h in the presence of 100 µM Asn, 1 µg ml−1 ASNase or PBS. The OT-I CD8+ T cells from OT-I mice were stimulated with 1 µg ml−1 OVA257–264(SIINFEKL) peptide.
Western blot analysis of TCR signalling in CD8+ T cells
Generally, CD8+ T cells were activated in medium (10% dialysed FBS) lacking Asn and Asp, supplemented with or without Asn at 37 °C for 30 min, and further stimulated with 10 μg ml−1 anti-CD3 and 1 μg ml−1 anti-CD28 antibodies for 15 min at 37 °C. The cells were then cooled on ice, followed by centrifugation at 3,000 r.p.m. for 4 min at 4 °C. The samples were analysed by western blotting.
Metabolic flux and LC–MS/MS analysis
Naive CD8+ T cells were seeded into 48-well plates in control medium (lacking Asn and Asp) or medium supplemented with 100 µM Asn or 4 mM 15N-Asp. After activation, the CD8+ T cells were washed twice with PBS buffer and subjected to cell counting using a TC20 automated cell counter (Bio-Rad). Metabolites were extracted using 100% acetonitrile and analysed using the Multi-reaction monitoring mode (MRM) of UPLC-QQQ-MS/MS (Agilent 1290/6460 tandem mass spectrum). An ACQUITY UPLC BEH HILIC, 2.1 mm × 100 mm, 1.7 µm (Waters) column was used for liquid chromatography separation. Gradient elution was performed using 0.1% formic acid acetonitrile (solvent A) and 0.1% formic acid water (solvent B). The settings were as follows: 0–1 min, 80% A; 1–5 min, 80 to 50% A; 5–7 min, 50% A; 7–7.1 min, 50 to 80% A; and 7.1–10 min, 80% A. The injection volume was set to 10 µl and the flow rate 0.4 ml min−1. Each sample was run for 10 min.
Metabolomics
Naive CD8+ T cells were isolated and activated at different time points in medium lacking Asn and Asp supplemented with or without Asn. The cells were then washed twice with ice-cold PBS and metabolites were extracted using ice-cold 80% methanol. The extracts were analysed by LC–MS/MS.
Protein expression and purification
PRK5–FLAG plasmids coding for the full-length mouse LCK, LCK(Y505F), LCK(Y394F), ZAP70 or SLP76 were transfected into 293T cells. After 48 h of transfection, the cells were harvested, lysed using lysis buffer (PBS, 1% Triton X-100 and proteinase inhibitors) on ice for 10 min and then centrifuged at 13,000 r.p.m. for 10 min at 4 °C. The supernatants were incubated with FLAG M2 beads, which were washed three times with wash buffer (PBS, 150 mM NaCl and 1% Triton X-100), followed by competitive elution with FLAG peptide PBS solution.
For purification of recombinant proteins from the Escherichia coli BL21 strain, PET-21B-strep-LCK, PET-21B-strep-LCK(Y394F) and PET-21B-strep-LCK(Y505F) were transformed into competent BL21 cells. The cells were then cultured in medium containing 200 g ml−1 ampicillin at 37 °C with shaking at 250 r.p.m. Protein expression was induced by adding IPTG to a final concentration of 1 mM at 18 °C for 18 h. The bacteria were collected and ultrasonicated in a lysis buffer containing 125 mM NaCl, 25 mM Tris–HCl (pH 7.5) and 1 mM PMSF. After centrifugation at 16,000 r.p.m. for 1 h at 4 °C, the supernatants were incubated with streptavidin-conjugated agarose beads for 4 h at 4 °C. The beads were then washed three times with lysis buffer. The bound proteins were eluted using Strep elution buffer (IBA, 2-1042-025).
Radiolabelled amino-acid binding assay
Purified proteins on beads were incubated with 3 µCi 3H-labelled amino acids and/or 10 mM cold amino acids for 20 min at room temperature. The tubes were gently shaken every 5 min during incubation. The beads were spun down briefly and washed three times with wash buffer (0.1% Triton X-100, 40 mM HEPES pH 7.4 and 150 mM NaCl), followed by drying and resuspension in 50 µl wash buffer. The suspension (10 µl) was added into an Isoplate-96-plate well and quantified using a MicroBETA2 scintillation counter (PerkinElmer). The remaining beads were then boiled in 50 µl sample buffer and resolved by 10% SDS–PAGE. The proteins were visualized by Coomassie blue staining.
BIAcore analysis
The purified proteins were immobilized on a Series S Sensor chip CM7 (GE Healthcare) in 10 mM sodium acetate buffer (pH 4.5). The amino acids were dissolved in PBS buffer to generate gradient concentrations in PBS running buffer. The mixtures were run across each sensor surface at a flow rate of 10 µl min−1 for a contact phase of 90 s, followed by a dissociation phase of 120 s in running buffer. The results were calculated using the BIAcore S200 Evaluation software Version 1.0 (GE Healthcare).
CD8+ T-cell cytotoxicity assay
OT-I CD8+ T cells were activated in medium lacking Asn and Asp supplemented with or without 100 µM Asn for 4 d. The cells were then cultured with pre-loaded B16-OVA or EG-7 cells for 18 h. The cytotoxic efficiency of the CD8+ T cells was measured using a lactate dehydrogenase A release assay using a microplate reader (Molecular Devices, SpectraMAX M2e).
Proliferation analysis by CFSE staining
The CFSE staining was performed according to the manufacturer’s manual (Invitrogen). Briefly, the isolated CD8+ T cells were resuspended in 0.1% BSA (in PBS) at a final concentration of 1–5 × 106 cells ml−1. The cells were incubated with CFSE solution (1 µM) at 37 °C for 10 min; the staining was stopped by adding five volumes of ice-cold culture medium and the cells were incubated for another 5 min on ice. The cells were spun down and washed at least twice with fresh medium. The cells were then loaded into a 48-well plate with Asn-free or -supplemented medium. After 3 d, the cells were analysed using flow cytometry.
Drug affinity responsive targets stability
Jurkat T cells were cultured in Asn-free medium for 12 h. Approximately 5 × 106 cells were lysed in 150 µl M-PER (Thermo Fisher, 78501) with 1×protease inhibitor cocktail. The cell lysates (100 µl) were transferred to a new tube, incubated with various concentration of Asn or PBS for 1 h at room temperature and digested with Pronase (6 µg ml−1) for 15 min at room temperature. After 15 min, the digestion was stopped by adding 5×SDS loading buffer and the samples were boiled at 98 °C for 10 min. Western blotting was used to determine the LCK protein levels.
Cellular thermal shift assay
Jurkat T cells were cultured in Asn-free medium for 12 h. The cells (5 × 106) cells were then pretreated with or without 400 μM Asn for 12 h before use in the cellular thermal shift assays assay. The cells were cooled on ice, washed with PBS containing protease inhibitor cocktail and transferred into PCR tubes. The cells were then heat shocked in a Bio-Rad T100 thermal cycler at the indicated temperature (60 to 40 °C) for 3 min to denature the proteins, and immediately cooled on ice. The cells were lysed by three freeze–thaw cycles with liquid nitrogen and a thermal cycler, centrifuged at 13,000 r.p.m. for 10 min at 4 °C and the supernatant was boiled with 5×SDS loading buffer for western blotting. The bands were quantified using the Image J software.
Translation assay by puromycin
Isolated naive CD8+ T cells were stimulated in the indicated medium with 3.5 µg ml−1 anti-CD3 and 1 μg ml−1 anti-CD28 antibodies for 24 h. Puromycin (40 µg ml−1) was then added 10 min before sample collection. The cells were stained by FITC-conjugated anti-puromycin antibody and puromycin incorporation was measured by FACS analysis.
Adoptive T-cell transfer
LmOVA were cultured in Brain Heart Infusion Medium (BHI). Briefly, the mice were immunized intravenously with recombinant attenuated LmOVA (2 × 105 colony-forming units (c.f.u.)). OT-I CD8+ T cells (CD45.2+; 2 × 105) were intravenously injected into the tail-vein of CD45.1+ mice, followed by LmOVA infection 1 d later. Splenocytes were isolated 7 d post OT-I CD8+ T-cell injection and analysed for the presence of OVA-specific CD8+ T cells by MHC class I tetramer (Kb/OVA257–264).
For the memory rechallenge experiments, mice maintained on a specific diet were immunized with attenuated LmOVA, followed by rechallenge with LmOVA (2 × 106 c.f.u.) 21 d after the primary immunization. Splenocytes were isolated 5 d post the rechallenge and analysed as described earlier.
In vivo priming of T cells for tumour experiments
Tumour cells (8 × 105) were subcutaneously injected into the dorsal region of C57BL/6 mice. The mice were immunodepleted by irradiation (2 Gy, twice) 5 d post injection. After another 24 h, 4 × 105 OT-I cells were adoptively transferred into these mice by tail-vein injection. The mice were immunized with SIINFEKL peptide (OVA257–264; 20 µg per mouse) in Freund’s complete adjuvant the following day. Asn (1 mmol per mouse), ASNase (0.2 mg per mouse) or PBS was intraperitoneally injected into the mice every 2 d, starting one day before immunization and lasting for a period of 14 d. During this period, the mice were maintained on a diet lacking Asn and Asp or normal diet. Animal survival was recorded daily. Tumour size (s) was calculated using the formula \(s = {\mathrm{\pi}} r^2\), where r is the radius of the tumour.
Tumour-infiltrating CD8+ T-cell responses
The tumour was digested with collagenase IV and the tumour-infiltrating leukocytes were isolated by 40–80% Percoll (GE Healthcare) gradient centrifugation. The isolated cells were stimulated with 50 ng ml−1 phorbol 12-myristate 13-acetate and ionomycin in the presence of 5 μg ml−1 Brefeldin A (BFA) for 4 h. The activation of CD8+ T cells were analysed by anti-mCD4, anti-mCD8 and anti-mCD3 ζ staining, followed by fixation using PBS buffer containing 4% paraformaldehyde and 0.01% Triton X-100. The fixed cells were subjected to anti-TNF-α, anti-IFN-γ, anti-GzmB and anti-Ki67 staining.
Lung-metastatic melanoma model
Mice were treated by γ-irradiation (2 Gy, twice) and received 4 × 105 OT-I cells by tail-vein injection 24 h later. The mice were immunized with SIINFEKL peptide (OVA257–264; 20 µg per mouse) in Freund’s complete adjuvant the next day. Asn (1 mmol per mouse) or PBS was intraperitoneally injected into the mice every 2 d, starting one day before immunization for a total of 14 d. During this period, the mice were fed a diet lacking Asn and Asp; otherwise, the animals were fed a normal diet. Animal survival was monitored. To study tumour growth, the mice were euthanized on day 17 and the metastatic tumours were counted. Lung-infiltrating T cells were isolated and analysed as described earlier.
Confocal immunofluorescence imaging
Imaging was performed on a custom modified Olympus FV1200 Laser Scanning Microscope equipped with a ×100 oil immersion lens. Briefly, naive CD8+ T cells were seeded in chambers and stimulated with 10 μg anti-CD3 and 1 μg anti-CD28 antibodies for 10 min at 37 °C. The cells were then fixed with 4% paraformaldehyde (PFA) and 0.1% Triton X-100 for 1 h at 4 °C, followed by 5% BSA blocking for 30 min at 37 °C. The cells were gently washed three times with PBS and stained overnight with the indicated antibody at 4 °C. After three washes with PBS, the cells were incubated with the secondary antibody for 1 h at 4 °C, and gently washed three times with PBS before imaging. The images were analysed using Image J.
Statistics and reproducibility
The experiments in this study were set up using 3–10 samples or animals per independent group, condition or repeat. Each experiment was repeated independently with similar results. Immunoblot detection, quantitative PCR with reverse transcription, kinase activity measurements, and the immunoprecipitation and immunofluorescence staining experiments were independently repeated at least three times. The FACS gating strategies are described in Extended Data Fig. 10. Representative dot plots for the FACS analysis are provided in the Supplementary figures. All of the data are presented as the mean ± s.e.m. and exact n values are indicated in the figure legends. The statistical analyses were performed using GraphPad Prism 7. Comparisons were analysed using an unpaired two-tailed Student’s t-test or a two-way ANOVA, followed by Tukey’s multiple comparisons test, as indicated in the corresponding figure legends. The P values are indicated in the related figures; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant (P > 0.05).
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
MacIver, N. J., Michalek, R. D. & Rathmell, J. C. Metabolic regulation of T lymphocytes. Annu Rev. Immunol. 31, 259–283 (2013).
Wang, R. & Green, D. R. Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915 (2012).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Rathmell, J. C., Vander Heiden, M. G., Harris, M. H., Frauwirth, K. A. & Thompson, C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 6, 683–692 (2000).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
O’Sullivan, D., Sanin, D. E., Pearce, E. J. & Pearce, E. L. Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 19, 324–335 (2019).
Wei, J., Raynor, J., Nguyen, T. L. & Chi, H. Nutrient and metabolic sensing in T cell responses. Front Immunol. 8, 247 (2017).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 482 (2017).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143 (2013).
Schluns, K. S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Best, J. A. et al. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat. Immunol. 14, 404–412 (2013).
Surh, C. D., Boyman, O., Purton, J. F. & Sprent, J. Homeostasis of memory T cells. Immunol. Rev. 211, 154–163 (2006).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 (2012).
Harty, J. T. & Badovinac, V. P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8, 107–119 (2008).
Krall, A. S., Xu, S., Graeber, T. G., Braas, D. & Christofk, H. R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 7, 11457 (2016).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338 (2012).
Meng, D. et al. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 295, 2890–2899 (2020).
Pavlova, N. N. et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 27, 428–438 (2018).
Brownlie, R. J. & Zamoyska, R. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269 (2013).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Kelly, B. & Pearce, E. L. Amino assets: how amino acids support immunity. Cell Metab. 32, 154–175 (2020).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Richards, N. G. & Kilberg, M. S. Asparagine synthetase chemotherapy. Annu. Rev. Biochem. 75, 629–654 (2006).
Deng, L. et al. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat. Commun. 11, 1755 (2020).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, eaau0135 (2019).
Acknowledgements
We thank X. Lin, L. Yu, X. Guo, L. Zhang, Y. Wang and X. Hu at Tsinghua University; H. Wu at Peking University; W. Du at Peking Union Medical College and C. Xu at Shanghai Institute of Biochemistry for materials and/or technical assistance. We thank H. Wang and W. Wu at Tsinghua University for their great support of this work during the COVID-19 pandemic. We thank all of the members of the Jiang laboratory for their technical assistance and/or discussions. We thank X. Liu, L. Xu, X. Wang and W. Wang for their help with the LC–MS/MS experiments. This research was supported by the National Key R&D Program of China (grant no. 2019YFA0801701), Tsinghua-Peking Center for Life Sciences and National Natural Science Foundation of China (grant nos 81930082 and 81722035) to P.J.
Author information
Authors and Affiliations
Contributions
J.W., G.L. and P.J. designed the experiments. J.W. and G.L. performed all of the experiments. L.L. and D.L. provided technical assistance. Z.D. provided constructive comments and supplied reagents. P.J. supervised the research and wrote the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks Vijay Kuchroo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro activation of CD8+ T cells by Asn.
a, 15N-Asp levels outside or within mouse naive CD8+ T cells activated in medium containing 4 mM 15N-Asp for 40 h. b-d, Surface expression of CD25 (b), CD44 (c), or CD69 (d) on CD8+ T cells stimulated in the presence of increasing amounts of Asn. e-g, Percentage of CD25 (e), CD44 (f), or CD69 (g) expression on CD8+ T cells stimulated with CD28 antibody (1 μg/ml) and increasing amounts of anti-CD3 antibody in the presence or absence of Asn. h, CFSE staining of mouse naive CD8+ T cells activated in Asn/Asp-free medium (Ctrl) or medium containing Asn for indicated time points. i and j, Proliferation of mouse CD8+ T cells treated as in (b) was determined by Ki67 staining (i), or BrdU incorporation assay (j). mFI, mean fluorescence intensity. k and l, Surface expression of CD25, CD44 and CD69 on mouse OT-I CD8+ T cells activated by OVA peptide in Asn/Asp-free medium (Ctrl) or Asn medium (k). Production of cytokines was also determined (l). m and n, Surface expression of CD69 (m) and CD44 (n) on mouse naive CD8+ T cells activated in Asn/Asp-free (Ctrl) or Asn medium containing 10% dialysed FBS. o-q, Surface expression of indicated proteins on mouse naive CD8+ T cells activated in Asn/Asp-free (Ctrl) or Asn medium containing no FBS. r and s, Representative flow cytometry plots of FSC-A versus TNF-α (r), or IFN-γ (s) staining for human CD8+ T cells treated as in Fig. 1k. Data are mean ± SEM, two-tailed Student’s t-test. **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. All experiments were done at least 3 times per group or condition. Related representative FACS plots (Supplementary Figs. 9, 10) and numerical source data for a-g, i, k-p are provided.
Extended Data Fig. 2 Effect of ASNase, Glu and Asn on CD8+ T cell responses.
a-d, Mouse CD8+ T cells cultured in RPMI 1640 medium, or medium containing 10 µg/ml ASNase were left untreated (naive) or stimulated with anti-CD3/CD28 antibodies for 4 h or 12 h. Relative levels of Asn, Asp, Gln and Glu within and outside cells were measured by LC-MS/MS. e and f, Mouse naive CD8+ T cells stimulated with anti-CD3/CD28 antibodies in control Glu-free medium (Ctrl) or medium containing Glu in the presence of dialysed FBS. Percentage of CD44+CD8+ T cells (e), CD62LlowCD44high CD8+ T cells and CD62LhighCD44high CD8+ T cells (f) relative to total CD8+ T cells were measured. g-j, Jurkat cells were stimulated with anti-CD3/CD28 antibodies in the absence or presence of L-Alb, PP2, or increasing amounts of Asn for 24 h (for detecting CD69 expression) or 40 h (for detecting CD25 expression). Surface expression of CD69 (c) or CD25 (d) was measured. Production of TNF-α (e), and IFN-γ (f) by the activated Jurkat cells was determined by FACS analysis. All data are mean ± SEM, n = 3 independent wells. Two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. Representative FACS plots for e-j (Supplementary Fig. 11) and numerical source data for a-j are provided.
Extended Data Fig. 3 Effect of extracellular and de novo Asn on T activation.
a, mRNA expression in naive CD8+ T cells. b and c, Mouse naive CD8+ T cells were left untreated (naive) or activated for different time points. ASNS expression (b) and Asn levels (c) were measured. d, Surface expression of CD25 and CD44 on pre-activated mouse naive CD8+ T cells cultured in Asn/Asp-free (Ctrl) or Asn medium. e-h, Mouse naive CD8+ T cells were activated as indicated. Intracellular (e) and extracellular (f) Asn levels, surface expression of CD25 and CD44 (g), and cytokine production (h) were measured. i and j, Survival (i) and apoptosis (j) of mouse naive CD8+ T cells activated in Asn/Asp-free (Ctrl) or Asn medium containing exogenous IL-2. k, Annexin V staining of naive CD8+ T cells activated in Asn/Asp-free (Ctrl) or Asn medium without exogenous IL-2. l-n, Naive CD8+ T cells pre-activated in 1640 IL-2-supplemented medium were cultured in IL-2-free medium without (Ctrl) or with Asn. Living cells (l), apoptotic (m) and proliferating (n) T cells were assayed at day 3 post IL-2 withdrawal. o and p, Mouse naive CD8+ T cells activated in ASNase-supplied or -free conditioned medium from H1299 cells or Asn/Asp-free medium (Ctrl) were analysed for activation and Asn levels. q, Asn levels in medium cultured from Gln-fed or starved H1299 and EG-7 cells. r, Mouse naive CD8+ T cells were stimulated in medium cultured from Gln-fed or starved EG-7 cells in the presence of 2 mM Gln. CM, conditioned medium. Data are mean ± SEM, n = 3 independent samples per group/condition. Two-tailed Student’s t-test or a two-way ANOVA followed by Sidak’s multiple comparisons (i). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Related representative FACS plots (Supplementary Fig. 12), uncropped blots for b, and numerical source data for a-m, o-r, are provided.
Extended Data Fig. 4 Increasing Asn promotes T cell activity in vivo.
a-j, C57BL/6 mice intraperitoneally injected with Asn or PBS (Ctrl) every two days for a period lasting one month. Spleens were excised, weighted and pictured (a). Relative numbers of cells in spleens and lymph nodes (b), weight of mice (c), Percentage of CD4+ T cells and CD8+ T cells relative to total cells in spleens and lymph nodes (d, e), effector (e) and central memory-like (f) CD8+ T cells relative to total CD8+ T cells in spleens and lymph nodes, CD25+CD4+ T cells (h), and effector (i) and central memory-like (j) CD4+ T cells relative to total CD4+ T cells in spleens and lymph nodes were assessed. k-r, C57BL/6 mice intraperitoneally injected with ASNase or PBS (Ctrl) every two days for a period lasting one month. Spleens were excised, weighted and pictured (k). Relative numbers of cells in spleens and lymph nodes (l), percentage of CD4+ T cells and CD8+ T cells relative to total cells in spleens and lymph nodes (m), effector memory-like (n) and central memory-like (o) CD8+ T cells relative to total CD8+ T cells in spleens and lymph nodes, percentage of CD25+CD4+ T cells (p), effector memory-like (q) and central memory-like (r) CD4+ T cells relative to total CD4+ T cells in spleens and lymph nodes were assessed. s, Surface expression of CD25 and CD44 on mouse naive CD8+ T cells activated with serum from normal diet mice intraperitoneally injected with PBS (Ctrl) or ASNase (n = 6 mice per group). All data are mean ± SEM, in a-r, Ctrl, n = 7 mice; Asn, n = 6 mice. Two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. Related representative FACS plots (Supplementary Figs. 13, 14) and numerical source data for a-s are provided.
Extended Data Fig. 5 Asn enhances OVA-induced CD8+ T cell activation in vivo and a role for SLC1A5 in Asn uptake in CD8+ T cells.
a-i, C57BL/6 mice orally administrated with normal (Ctrl) or Asn-supplemented water (Asn) were infected with LmOVA for 7 days (a). spleens were excised, weighted and pictured (b, c). Percentage of CD8+ T cells and CD4+ T cells (d-g), and OVA-TET+ CD8+ T cells in spleens and lymph nodes (h, i) were determined. n = 5 mice per group. j-l, Related to Fig. 2m,n. Asn-free diet CD45.1 mice intraperitoneally injected with PBS (Ctrl) or Asn were adoptively transferred with naive CD45.2+ OT-I CD8+ T cells and injected with B16-OVA cells as indicated (j). 16 days later, percentage and number (#) of CD44+CD8+ T cells (k), and percentage of central and effector memory-liked CD8+ T cells relative to total CD8+ T cells in lymph nodes (l) were analysed (n = 5 mice per group). m, mRNA levels of potential transporters for Asn uptake and exchange in Jurkat cells. n and o, SLC1A5 and SLC38A2 expression in mouse naive CD8+ T cells activated in Asn/Asp-free (Ctrl) or Asn medium was analysed by qRT-PCR (n) and western blotting (o). p, Mouse CD8+ T cells activated by anti-CD3(5 μg/ml) and anti-CD28(1 μg/ml) in Asn/Asp-free (-) or Asn medium supplied with 10% dialysed FBS and 2 mM Gln were treated with or without 17 μM V9302 for 12 h. Surface expression of CD25 and CD69 relative to total CD8+ T cells were quantified. All data are mean ± SEM. Data in o are representative of three independent experiments, two-tailed Student’s t-test. *P < 0.05; ***P < 0.001; ***P < 0.001; ***P < 0.0001; ns, not significant. Related representative FACS plots (Supplementary Fig. 15), uncropped blots for o, and numerical source data for c-i, k-n, p, are provided.
Extended Data Fig. 6 Effect of mTORC1 and Gln on Asn-mediated CD8+ T cell activation.
a, Phosphorylation of the mTORC1 effector S6 (phospho-S6 Ser 235/236) in mouse CD8+ T cells left unstimulated (naive) or activated in the presence of increasing amount of Asn. b, Western blot analysis of mouse naive CD8+ T cells left untreated (naive), or activated in Ctrl (Asn/Asp-free) medium or Asn medium supplemented with increasing amounts of rapamycin for 12 h. c, Surface expression of CD69, CD25, and CD44 on mouse naive CD8+ T cells stimulated in Asn/Asp-free medium (Ctrl) or Asn medium with 10% dialysed FBS and increasing amounts of rapamycin (n = 3 independent wells). d, Mouse naive CD8+ T cells stimulated in Asn/Asp-free (-) or Asn-supplied (Asn) medium without (0) or with increasing amounts of Gln for 24 h were treated with 40 μg/ml Puromycin for another 10 min. Medium was added with 10% dialysed FBS. Puromycin incorporation was measured by FACS analysis using FITC-conjugated anti-Puromycin antibody (n = 3 independent wells). Representative flow cytometry plots are shown. e, Mouse naive CD8+ T cells were stimulated in the indicated medium with 10% dialysed FBS for 24 h. MG132 (10 μM) was added 2 h before harvesting for western blot analysis. f, Surface expression of CD69, CD25, and CD44 on mouse naive CD8+ T cells stimulated in Asn/Asp-free (-) or Asn medium containing no (0) or increasing amounts of Gln for 24 h (n = 3 independent wells). All medium was added with 10% dialysed FBS. All data are mean ± SEM, two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ***P < 0.0001; ns, not significant. Data in a, b and e are representative of three independent experiments. Related representative FACS plots (Supplementary Figs. 16, 17), uncropped blots for a, b, e, and numerical source data for c, d, f, are provided.
Extended Data Fig. 7 Impact of Asn on CD8+ T cell metabolism and TCR signalling.
a and b, Mouse naive CD8+ T cells were left untreated (naive) or activated in Asn/Asp-free medium (Ctrl), or medium supplied with Asn or Asp for indicated times. All medium was added with 10% dialysed FBS. The abundance of cellular metabolites at different time points were analysed by LC-MS/MS. The heat map shows the relative abundance of metabolites over time in CD8+ T cells stimulated in Ctrl, Asn- or Asp-medium. Log2 fold changes (FC) are relative to relative to cells stimulated in Asn/Asp-free (Ctrl) medium at corresponding time points. Data are representative of three independent experiments. Structural isomers were condensed and counted only once as the employed technology cannot distinguish metabolites with identical molecular weight. c, TCR signalling that initiates T cell activation. d, Related to Fig. 4e. Scheme of OT-I adoptive transfer and OVA-peptide treatment experiment. e, Western blot analysis of mouse CD8+ T cells activated in medium containing increasing amounts of Asn in the absence or presence of PP2 (10 µM). Data are representative of three independent experiments. f, Related to Fig. 5e–k. CD45.1 C57BL/6 mice maintained on an Asn-free diet were intraperitoneally injected with Asn or PBS (Ctrl), or PP2 (30 µg/mouse) or GDC-0032 (100 µg/mouse) every two days. 8 days later, mice were adoptively transferred with naive CD45.2+ OT-I CD8+ T cells and injected with OVA peptide as indicated. g, Related to Fig. 5l–o, Scheme of Asn-free diet CD45.1 C57BL/6 mice i.p. with Asn and/or PP2 and then adoptively transferred with naive CD45.2+ OT-I CD8+ T cells and infected with LmOVA as indicated. Uncropped blots for e and numerical source data for a, b, are provided.
Extended Data Fig. 8 LCK is a natural sensor for Asn.
a, Recombinant LCK proteins were treated with ATP and Asn or Asp. LCK kinase activity was determined by ELISA. b and c, BIAcore measurement of the interaction between Asn or Asp and purified mutant LCK (Y394F) (b) or LCK (Y505F) (c). d-u, Screening the binding specificity of recombinant LCK proteins to different amino acids by BIAcore assay. Graphs of equilibrium response units (RU) and compound concentrations are shown. Gln, glutamine; Thr, threonine; Isoleu, isoleucine; Trp, tryptophan; Met, methionine; Leu, leucine; Ala, alanine; Val, valine; Ser, serine; Pro, proline; Gly, glycine; His, histidine; Cys, cysteine; Tyr, tyrosine; Phe, phenylalanine; Lys, lysine; Glu, glutamate; Arg, arginine. v, BIAcore assay of the interaction between purified ZAP70 proteins and Asn or Asp. w, CETSAs exhibit the binding affinity of LCK to Asn, but not Gln, in Jurkat T cells. Relative LCK band intensities were quantified and plotted against corresponding incubation temperatures. x, Direct binding of LCK to Asn, but not Gln in Jurkat cells measured by DARTS assays. y, In vitro kinase assay for LCK and its mutants in the presence of increasing amounts of Asn. Kinase activity is expressed as relative values to phosphorylation of LCK(Y394). z, In vitro kinase assay using purified LCK and its mutants left untreated (Ctrl), or incubated with ATP and increasing amounts of Asn for 15 min. In b-v, Graphs of equilibrium response units (RU) and compound concentrations are shown. In b, c and v, Purified proteins were analysed by SDS-PAGE followed by Coomassie Blue staining. Data are mean ± SEM, in a, n = 3 biological replicates. Two-way ANOVA followed by Sidak’s multiple comparisons. Data are representative of three independent experiments throughout. Uncropped blots for x, z, and numerical source data for a-w, y, are provided.
Extended Data Fig. 9 Asn binds to the LCK kinase domain and enhances CD8+ T cell anti-toumor activity.
a and b, BIAcore measurement of the interaction between Asn and purified region AA220-390 (a) or AA320-509 (b). Graphs of equilibrium response units (RU) and compound concentrations are shown. c, Mapping the Asn binding site on LCK. The region responsible for Asn binding and results of Asn binding analysis are shown. d, OVA-specific OT-I CD8+ T cells pre-activated in Asn/Asp-free (Ctrl) or Asn medium were cultured with B16-OVA cells for 18 h. B16-OVA cell apoptosis was analysed (n = 3 independent wells). e-p, C57BL/6 mice maintained on a normal (Ctrl) or Asn diet (e-j), or on a normal diet yet subcutaneously injected with PBS (Ctrl) or ASNase (0.2 mg/mouse) were subcutaneously injected with B16-F10 cells near the inguinal lymph node for 12 days. Percentage of CD44+CD8+ T cells (e, k), effector (f, l) and central memory-like (g, m), naive (h, n) CD8+ T cells, and production of cytokines (i, j, o, p) in lymph nodes were measured (n = 7 or 8 mice per group). q, Related to Fig. 7b and c. Schematic view of the B16-OVA and activated OT-I CD8+ T cell adoptive transfer experiment. r, Naive OT-I CD8+ T cells primed in vitro in Asn or Asn/Asp-free medium (Ctrl) were injected into C57BL/6 mice subcutaneously injected (sc) with B16-OVA cells as indicated. Tumour burden were analysed over time. Data are representative of three independent experiments (n = 5 mice per group). s, Schematic of the experimental approach in Fig. 7h–j. All data are mean ± SEM. In d-p, two-tailed Student’s t-test, and in r, two-way ANOVA followed by Sidak’s multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. Related representative FACS plots (Supplementary Fig. 18) and numerical source data for a, b, d-p, r, are provided.
Extended Data Fig. 10 Gating strategies used in FACS analysis.
a, Gating strategy to identify APC+ in mouse spleen isolated CD8+ T cells which were activation such as Fig. 1a and Supplemental Fig. 1b. b, Gating strategy to analyse CFSE-stained or BrdU-stained activation CD8+ T cells analysis presented on Extended Data Fig. 1h,1j and 3n. c, Gating strategy to analyse annexin V stained activation CD8+ T cells in apoptosis analysis presented on Extended Data Fig. 3k,m. d, Gating strategy to analyse the population of CD4+ T cells or CD8+ T cells in mouse lymph nodes or spleens presented on such as Extended Data Fig. 4m. e, Gating strategy to analyse the population of OT-I CD8+ T cells in mouse lymph nodes or spleens presented on such as Figs. 2m, 2n, 5e–o, and Extended Data Fig. 5k,l.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18
Supplementary Tables 1–3
Supplementary Table 1. Information about the antibodies used in this work. Supplementary Table 2. List of the primer pairs used in this study. Supplementary Table 3. Information about the human participants.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 4
Uncropped gels
Source Data Fig. 5
Statistical source data
Source Data Fig. 5
Uncropped gels
Source Data Fig. 6
Statistical source data
Source Data Fig. 6
Uncropped gels
Source Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data
Source Data Extended Data Fig. 2
Statistical source data
Source Data Extended Data Fig. 3
Statistical source data
Source Data Extended Data Fig. 3
Uncropped gels
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 5
Uncropped gels
Source Data Extended Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 6
Uncropped gels
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 7
Uncropped gels
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 8
Uncropped gels
Source Data Extended Data Fig. 9
Statistical source data
Rights and permissions
About this article
Cite this article
Wu, J., Li, G., Li, L. et al. Asparagine enhances LCK signalling to potentiate CD8+ T-cell activation and anti-tumour responses. Nat Cell Biol 23, 75–86 (2021). https://doi.org/10.1038/s41556-020-00615-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00615-4
This article is cited by
-
Metabolism of asparagine in the physiological state and cancer
Cell Communication and Signaling (2024)
-
Cancer immunometabolism: advent, challenges, and perspective
Molecular Cancer (2024)
-
Metabolic waypoints during T cell differentiation
Nature Immunology (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Amino acid metabolism in tumor biology and therapy
Cell Death & Disease (2024)