Abstract
Aberrant epithelial reprogramming can induce metaplastic differentiation at sites of tissue injury that culminates in transformed barriers composed of scar and metaplastic epithelium. While the plasticity of epithelial stem cells is well characterized, the identity and role of the niche has not been delineated in metaplasia. Here, we show that Gli1+ mesenchymal stromal cells (MSCs), previously shown to contribute to myofibroblasts during scarring, promote metaplastic differentiation of airway progenitors into KRT5+ basal cells. During fibrotic repair, Gli1+ MSCs integrate hedgehog activation signalling to upregulate BMP antagonism in the progenitor niche that promotes metaplasia. Restoring the balance towards BMP activation attenuated metaplastic KRT5+ differentiation while promoting adaptive alveolar differentiation into SFTPC+ epithelium. Finally, fibrotic human lungs demonstrate altered BMP activation in the metaplastic epithelium. These findings show that Gli1+ MSCs integrate hedgehog signalling as a rheostat to control BMP activation in the progenitor niche to determine regenerative outcome in fibrosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The mouse RNA-seq data reported in this paper (Fig. 4) are deposited in the NCBI Gene Expression Omnibus (GEO) under the accession number GSE140032. The human RNA-seq data reported in this paper (Fig. 7) are deposited in the NCBI GEO under the accession number GSE132771. Previously published sequencing data from the Tabula Muris cellular database were referenced here and are publicly available at https://tabula-muris.ds.czbiohub.org/. There are no restrictions on data availability. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
This study does not include any custom code.
References
Giroux, V. & Rustgi, A. K. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 17, 594–604 (2017).
Chilosi, M. et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of ΔN-p63. Lab. Invest. 82, 1335–1345 (2002).
Seibold, M. A. et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS ONE 8, e58658 (2013).
Xu, Y. et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1, e90558 (2016).
Prasse, A. et al. BAL cell gene expression is indicative of outcome and airway basal cell involvement in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 199, 622–630 (2019).
Hogan, B. L. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Ray, S. et al. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Rep. 7, 817–825 (2016).
Vaughan, A. E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Yang, Y. et al. Spatial–temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev. Cell 44, e754 (2018).
Yee, M. et al. Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells. Am. J. Respir. Cell Mol. Biol. 56, 453–464 (2017).
Peng, T. et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526, 578–582 (2015).
Zhao, H. et al. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 17, 386–396 (2015).
Zhao, H. et al. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14, 160–173 (2014).
Kramann, R. et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015).
Wang, C. et al. Expansion of hedgehog disrupts mesenchymal identity and induces emphysema phenotype. J. Clin. Invest. 128, 4343–4358 (2018).
Xi, Y. et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 19, 904–914 (2017).
Degryse, A. L. et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L442–L452 (2010).
Kurche, J. S. et al. Muc5b enhances murine honeycomb-like cyst formation. Am. J. Respir. Cell Mol. Biol. 61, 544–546 (2019).
Jeong, J., Mao, J., Tenzen, T., Kottmann, A. H. & McMahon, A. P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937–951 (2004).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
The Tabula Muris Consortium et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Zagorski, M. et al. Decoding of position in the developing neural tube from antiparallel morphogen gradients. Science 356, 1379–1383 (2017).
Massague, J., Seoane, J. & Wotton, D. Smad transcription factors. Genes Dev. 19, 2783–2810 (2005).
Plantier, L. et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax 66, 651–657 (2011).
Tadokoro, T., Gao, X., Hong, C. C., Hotten, D. & Hogan, B. L. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 143, 764–773 (2016).
Mou, H. et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217–231 (2016).
Bolanos, A. L. et al. Role of sonic hedgehog in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 303, L978–L990 (2012).
Jia, G. et al. CXCL14 is a candidate biomarker for Hedgehog signalling in idiopathic pulmonary fibrosis. Thorax 72, 780–787 (2017).
Lee, J. et al. Increased primary cilia in idiopathic pulmonary fibrosis. Mol. Cells 41, 224–233 (2018).
Liu, L. et al. Hedgehog signaling in neonatal and adult lung. Am. J. Respir. Cell Mol. Biol. 48, 703–710 (2013).
Moshai, E. F. et al. Targeting the hedgehog-glioma-associated oncogene homolog pathway inhibits bleomycin-induced lung fibrosis in mice. Am. J. Respir. Cell Mol. Biol. 51, 11–25 (2014).
Koli, K. et al. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am. J. Pathol. 169, 61–71 (2006).
Myllarniemi, M. et al. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177, 321–329 (2008).
Dong, Y. et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J. Exp. Med. 212, 235–252 (2015).
Butler, A. et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Acknowledgements
We thank K. N. Cheong, A. Brumwell, A. Baldwin and N. Reyes de Mochel for providing technical assistance; C. Gralapp for assistance with model illustration; M. Looney for critical review of manuscript; the Parnassus Flow Cytometry Core for assistance with cell sorting for bulk and single-cell RNA analysis (P30DK063720); members of the Biological Imaging Development Core (P30 DK063720); and E. Wan and the Institute for Human Genetics Core for the processing of single-cell RNA samples and high-throughput sequencing. This work is supported by NIH grants DP2AG056034, K08HL121146 and R01HL142552 to T.P., along with a Tobacco Related Disease Research Program New Investigator Award and a Pulmonary Hypertension Association award to T.P. and a Nina Ireland Program Award to M.M. for human lung collection.
Author information
Authors and Affiliations
Contributions
M.C. and T.P. conceived the experiments. M.C., C.W., J.K., T.T., P.M., M.M. and P.W. performed the experiments and data analyses. D.S., A.M. and H.C. provided expertise and feedback. M.C. and T.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
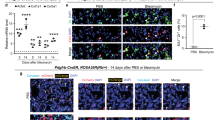
Extended Data Fig. 1 Epithelial progenitors expand distally in fibrotic repair.
(a, b) Time course showing appearance of metaplastic airway epithelium in the alveoli after 1, 2, 3 or 4 doses of weekly bleomycin injury. (n = 3 per group; each data point represents one animal). Data are expressed as mean ± SD. (c, d) Contribution of Sox2 Lin+ cells to the various epithelial lineages in the alveoli after bleomycin injury. (n = 6 per group; each data point represents one animal; one-tailed unpaired Student’s t-test). Data are expressed as mean ± SD. (e) Lack of Sox2 Lin+ cells in corn oil-treated SoxcreERT2:R26mTmG lung. (2 animals). (f) Co-localization of Sox2 Lin+ cells and SOX2 staining. (n = 3 animals). Data are expressed as mean ± SD. (g) Extended washout after tamoxifen (>4wks) followed by bleomycin demonstrates similar expansion of Sox2 Lin+ cells into the alveoli (3 animals). AW = airway. Scale bars, 100 μm.
Extended Data Fig. 2 Lineage tracing of diverse airway progenitors after bleomycin injury.
(a, b) p63 Lin+ cells also expanded into the alveoli after bleomycin but did not contribute significantly to KRT5 + basal cells in the alveoli. (n = 3 animals). Data are expressed as mean ± SD. (c, d) Comparison of p63 Lin+ and Sox2 Lin+ cells that expand into the alveoli after bleomycin injury. (n = 3 for p63 Lin, n = 6 for Sox2 Lin; each data point represents one animal; one-tailed unpaired Student’s t-test). Data are expressed as mean ± SD. (e, f) Contribution of Scgb1a1 Lin+ cells to the various epithelial lineages in the alveoli after bleomycin injury. (n = 3 per group; each data point represents one animal). Data are expressed as mean ± SD. AW = airway. Scale bars, 100 μm.
Extended Data Fig. 3 Gli1 Lin + mesenchyme expands distally in fibrotic repair.
(a) Lack of Gli1 Lin+ cells in corn oil-treated Gli1creERT2:R26YFP lung (2 animals). (b, c) Comparison of Gli1 Lin+ cell localization with Gli1 + cells in Gli1LacZ/+ lung. These experiments were each repeated three times independently with similar results. (d, e) Extended washout after tamoxifen (>4wks) followed by bleomycin demonstrates similar expansion of Gli1 Lin+ cells into the alveoli. (n = 3 per group; each data point represents one animal; one-tailed unpaired Student’s t- test). Data are expressed as mean ± SD. (f, g) Deletion of Gli1 + cells does not alter airway epithelial composition during normal homeostasis. (n = 3 per group; each data point represents one animal; one-tailed unpaired Student’s t-test). Data are expressed as mean ± SD. (h–j) Gli1-deleted lungs treated with bleomycin demonstrate reduced expression of Gli1 along with changes in fibrotic markers (n = 6 for control, n = 5 for Gli1- deleted; each data point represents one animal; for (i), Col1a1 P = 0.0862; Snai1 P = 0.3126; Cdh2 P = 0.0310; one-tailed unpaired Student’s t-test for (h–j)). Data are expressed as mean ± SD. (k) Weight loss after bleomycin injury in control and Gli1- deleted animals (n = 6 for control, n = 5 for Gli1-deleted; each data point represents one animal; one-tailed unpaired Student’s t-test). Data are expressed as mean ± SD. AW = airway. Scale bars, 100 μm.
Extended Data Fig. 4 ITGB4 + airway progenitors give rise to KRT5 + and SFTPC + cells that are not from pre- existing Krt5 + and Sftpc + cells.
(a) Gating strategy for isolation of ITGB4 + airway progenitors and Gli1 + mesenchymal cells by FACS, presented in Fig. 2a, e; 3 f; 5e; 6 f and Extended Data Fig. 4d, h; 5h; 8g. (b) Cytospin of freshly sorted ITGB4 + confirms that majority of cells are SOX2 + , SCGB1A1 + , and TubIV + , with cells rarely positive for SFTPC, KRT5, p63, or NGFR (n = 1 for NGFR, n = 3 for rest; each data point represents one biological replicate). Data are expressed as mean ± SD. (b) Z-axis scan shows that Gli1 Lin+ mesenchyme settle to bottom of the Matrigel well. (d–g) Lineage trace of pre-existing Krt5 Lin + (tdTomato + ) cells followed by isolation of lung for ITGB4 + airway progenitor organoid culture with Gli1 + cells. Tracheal Krt5 Lin+ cells demonstrate expression of basal cell markers KRT5 and p63 (e), while few Krt5 Lin+ cells appear in ITGB4 + cell-derived organoid isolated from the lung of the same animal (f, g). The overwhelming majority of KRT5 + cells in the ITGB4-derived organoids are Krt5 Lin-negative. (n = 3 wells for (f, g); n = 1 sample for (e)). Data are expressed as mean ± SD. (h–j) Lineage trace of pre-existing Sftpc Lin + (membrane GFP + ) cells followed by isolation of the lung epithelium for organoid culture with Gli1 + cells. ITGB4 + (red box) or Sftpc Lin + (green box) cells were sorted from the same lung. The overwhelming majority of SFTPC + organoids in the ITGB4- derived organoids are Sftpc Lin-negative, while most SFTPC + cells in the Sftpc Lin+ derived organoids are Lin + (n= 3; each data point represents one well). Data are expressed as mean ± SD. Scale bars, 100 μm.
Extended Data Fig. 5 Characterization of Hh- activation during homeostasis and in fibrotic repair.
(a) RNAScope in situ of Shh shows expression in ectopic SOX2 + cells in the alveoli after bleomycin injury. This experiment was repeated independently twice with similar results. (b, c) Histology quantification shows no evidence of KRT5 + metaplasia in Hh-activated animals without injury when compared to controls (n = 3 per group; each data point represents one animal; one-tailed unpaired Student’s t- test). Data are expressed as mean ± SD. (d–g) After bleomycin injury, Hh-activated lungs show similar expansion of Gli1 + cells in distal alveoli compared to controls, a trend towards increased myofibroblasts (SMA + ) differentiation of the Gli1 Lin+ cells in the alveoli, and no difference in ectopic SCGB1A1 + cells in the alveoli (for (e), n = 4 for control, n = 6 for Hh-activated; for (f, g), n = 9 per group; each data point represents one animal; one-tailed unpaired Student’s t-test for (e–g)). Data are expressed as mean ± SD. (h) Model of 3D airway organoid assay using Hh-inducible mesenchyme whereby pretreatment of 4- hydroxytamoxifen (4OHT) induces Hh activation in the mesenchyme as shown by upregulation of Gli1 transcript compared to vehicle (ethanol) (n = 3 per group; each data point represents one biological replicate; one-tailed unpaired Student’s t-test). (i–l) Mesenchymal Hh activation in vitro reduces CFE and organoid size, but increases the expression of Krt5 and number of KRT5 + organoids while reducing Sftpc expression and number of SFTPC + organoids ((i–k) n = 3 per group; (l) n = 3 for Hh-activated, n = 5 for control; each data point represents one well; one-tailed unpaired Student’s t-test for (j–l)). Data are expressed as mean ± SD. Hh = hedgehog, 4OHT = 4-hydroxytamoxifen, AW = airway. Scale bars, 100 μm.
Extended Data Fig. 6 Expansion of Gli1 Lin + mesenchyme into the alveoli.
(a) Multi-colour stochastic recombination of Gli1 Lin+ cells tagged with one of four possible fluorescent proteins (GFP, YFP, RFP, or CFP) shows clones of single color clustering around the airway in PBS treated lungs, and clones of single color spanning the airway and alveoli following bleomycin injury (4 animals per group) (b) Violin plots showing retention of Gli1 expression in Gli1 Lin+ cells following bleomycin injury as they migrate into the alveoli. Each black dot represents one cell (n values listed in source data for Fig. 4d). AW = airway. Scale bars, 100 μm.
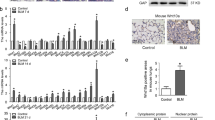
Extended Data Fig. 7 Spatial regulation of BMP activation.
(a, b) pSMAD1/5/8 staining of Sox2 Lin+ cells in the alveoli after bleomycin injury demonstrates that the majority of pSMAD1/5/8 + /Sox2 Lin+ cells are SCGB1A1 + or SFTPC + , and rarely KRT5 + (n = 3 for KRT5 quantification, n = 4 for SCGB1a1 and SFTPC quantification; each data point represents one animal). Data are expressed as mean ± SD. (c) qPCR of Hh-inducible cultured mesenchyme isolated from UbccreERT2/+:R26SmoM2/+ lungs treated with 4OHT + /-TGF-β, showing effect on various BMP antagonists expression (n = 3; each data point represents one biological replicate). Wnt5a: Control v TGF-β P = 0.0094, Control v Hh-activated P = 0.1066, Control v TGF-β + Hh-activated P= 0.002; Fst: Control v TGF-β P = 0.0598, Control v Hh- activated P = 0.0389, Control v TGF-β + Hh-activated P = 0.3833; Fstl1: Control v TGF-β P = 0.2051, Control v Hh- activated P = 0.4620, Control v TGF-β + Hh-activated P = 0.028; Fstl3: Control v TGF-β P = 0.0005, Control v Hh- activated P = 0.0704, Control v TGF-β + Hh-activated P = 0.0003; Grem1: Control v TGF-β P = 0.4845, Control v Hh- activated P = 0.2422, Control v TGF-β + Hh-activated P = 0.1417; Fzd1: Control v TGF-β P = 0.3341, Control v Hh- activated P = 0.2689, Control v TGF-β + Hh-activated P = 0.3320; Sfrp1: Control v TGF-β P = 0.4103, Control v Hh- activated P = 0.4614, Control v TGF-β + Hh-activated P = 0.1093; ordinary one-way ANOVA test. Data are expressed as mean ± SD. Scale bars, 100 μm.
Extended Data Fig. 8 Effect of rhBMP4 in vivo and in vitro.
(a–d) Effect of rhBMP4 treatment on fibrotic marker expression, myofibroblasts in the alveoli, weight change, and presence of ectopic SCGB1A1 + cells, in bleomycin injured C57BL/6 animals (n = 8 per group; each data point represents one animal). For (a), Col1a1 P = 0.0015; Snai1 P= 0.0769; Cdh2 P = 0.1771; Acta2 P = 0.0934; Tagln P = 0.3782.; one-tailed unpaired Student’s t-test for (a–d). Data are expressed as mean ± SD. (e) Effect of rhBMP4 on the expression of markers of chondrogenesis, adipogenesis, and osteogenesis in the lung (n = 8 per group; each data point represents one animal). Acan P = 0.0021; Col2a1 P = 0.2363; Col10a1 P = 0.4201; Plin2 P = 0.0578; Pparg P = 0.4343; Fabp4 P = 0.0006; Spp1 P = 0.3117; Alpl P = 0.0474; Ibsp P = 0.3371; Runx2 P < 0.0001; one-tailed unpaired Student’s t-test. Data are expressed as mean ± SD. (f) Effect of rhBMP4 on the total number of Sox2 Lin+ cells in the alveoli after bleomycin injury (n = 4 per group; each data point represents one animal; one-tailed unpaired Student’s t-test). Data are expressed as mean ± SD. (g–i) Airway progenitor organoid co-cultured with Gli1 + mesenchyme treated with BMP4 demonstrates increased CFE and enhanced Sftpc expression with reduced Krt5 (n = 4 for solvent, n = 3 for BMP4; each data point represents one well; one-tailed unpaired Student’s t-test for (h, i)). Conversely, the BMP antagonist GREM2 acts to suppress CFE and Sftpc expression while enhancing Krt5 (n = 4 per group; each datapoint represents one well; one-tailed unpaired Student’s t-test for (h,i)). Data are expressed as mean ± SD.
Extended Data Fig. 9 Metaplastic KRT5 + basal cells and SMA + fibroblastic foci in the alveoli of IPF lungs.
(a) UMAP projection of mesenchyme from each individual donor that was used in single cell RNA-seq analysis with contributing cell number. (b) Expression of basal cell markers NGFR, p63, and KRT14, in KRT5 + basal cells in human IPF, along with mouse bleomycin injured lungs and murine airway progenitor- derived organoids. This experiment was repeated three times independently with similar results. (c) Ectopic expression of KRT5 and SMA in the alveoli of IPF lungs form honeycomb cysts and fibroblast foci, respectively. This experiment was repeated independently twice with similar results. (d) Average cell-to-cell distance shows equivalent proximity of metaplastic KRT5 and endogenous SFTPC cells to SMA + fibroblastic foci (n = 3,280 KRT5 cells, n = 2,215 SFTPC cells; each datapoint represents an individual distance measurement with all measurements plotted for 3 samples; one-tailed unpaired Student’s t-test). (e, f) Unsorted epithelial organoids derived from dissociated IPF lungs grew KRT5 + organoids, and BMP4 attenuated the number and size of IPF-derived organoids. (n = 5; each data point represents one well; one-tailed unpaired Student’s t-test) Data are expressed as mean ± SD. Scale bars, 100 μm.
Extended Data Fig. 10 Gli1 + mesenchyme modulates SOX2 + progenitor fate.
Model of Gli1 + mesenchyme integrating Hh activation as a rheostat that controls local BMP activation to determine SOX2 + airway epithelial progenitor fate.
Supplementary information
Supplementary Table 1
List of qPCR primers (mouse) used in this study.
Source data
Source Data Fig. 1
Statistical source data for histological quantifications.
Source Data Fig. 2
Statistical source data for histological quantifications.
Source Data Fig. 3
Statistical source data for histological quantifications and arterial oxygen measurement raw data.
Source Data Fig. 4
Statistical source data for cluster information and Gene Ontology raw data.
Source Data Fig. 5
Statistical source data for histological quantifications and qPCR.
Source Data Fig. 6
Statistical source data for histological quantifications, arterial oxygen measurements and qPCR.
Source Data Fig. 7
Gene Ontology raw data and statistical source data for histological quantifications.
Source Data Extended Data Fig. 1
Statistical source data for histological quantifications.
Source Data Extended Data Fig. 2
Statistical source data for histological quantifications.
Source Data Extended Data Fig. 3
Statistical source data for histological quantifications, qPCR and weight change.
Source Data Extended Data Fig. 4
Statistical source data for histological quantifications.
Source Data Extended Data Fig. 5
Statistical source data for histological quantifications and qPCR.
Source Data Extended Data Fig. 7
Statistical source data for histological quantifications and qPCR.
Source Data Extended Data Fig. 8
Statistical source data for histological quantifications, qPCR and weight change.
Source Data Extended Data Fig. 9
Statistical source data for histological quantifications.
Rights and permissions
About this article
Cite this article
Cassandras, M., Wang, C., Kathiriya, J. et al. Gli1+ mesenchymal stromal cells form a pathological niche to promote airway progenitor metaplasia in the fibrotic lung. Nat Cell Biol 22, 1295–1306 (2020). https://doi.org/10.1038/s41556-020-00591-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00591-9
This article is cited by
-
Improved therapeutic consistency and efficacy of CD317+ MSCs through stabilizing TSG6 by PTX3
Stem Cell Research & Therapy (2024)
-
The functional effects of Piezo channels in mesenchymal stem cells
Stem Cell Research & Therapy (2023)
-
Gli1 marks a sentinel muscle stem cell population for muscle regeneration
Nature Communications (2023)
-
Abnormal respiratory progenitors in fibrotic lung injury
Stem Cell Research & Therapy (2022)
-
GREM1/PPP2R3A expression in heterogeneous fibroblasts initiates pulmonary fibrosis
Cell & Bioscience (2022)