Abstract
Redox balance, an essential feature of healthy physiological steady states, is regulated by circadian clocks, but whether or how endogenous redox signalling conversely regulates clockworks in mammals remains unknown. Here, we report circadian rhythms in the levels of endogenous H2O2 in mammalian cells and mouse livers. Using an unbiased method to screen for H2O2-sensitive transcription factors, we discovered that rhythmic redox control of CLOCK directly by endogenous H2O2 oscillations is required for proper intracellular clock function. Importantly, perturbations in the rhythm of H2O2 levels induced by the loss of p66Shc, which oscillates rhythmically in the liver and suprachiasmatic nucleus (SCN) of mice, disturb the rhythmic redox control of CLOCK function, reprogram hepatic transcriptome oscillations, lengthen the circadian period in mice and modulate light-induced clock resetting. Our findings suggest that redox signalling rhythms are intrinsically coupled to the circadian system through reversible oxidative modification of CLOCK and constitute essential mechanistic timekeeping components in mammals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data that support the findings of this study have been deposited in the Sequence Read Archive (SRA) under accession code PRJNA449625. Previously published ChIP-seq data and crystal structure that were reanalysed here are available in the Gene Expression Omnibus (GEO) under accession code GSE39860 (ref. 36) and in the Protein Data Bank (PDB) under accession code 4F3L (ref. 26), respectively. Mass spectrometry data generated for the H2O2-sensitive TF screen and mass spectrometry data for purified CLOCK protein have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) through the iProX partner repository with the dataset identifiers PXD015265 and PXD015266, respectively. Source data are available online for Figs. 1b,c,e–g, 2e,g,i–k, 3b,c,g,l, 4c–e,g–n, 5a–k, 6a,b,f–k and 7b,c,e,g and Extended Data Figs. 1b,c, 2f–h, 3b,e, 4h,l, 5a,c,e,g–i,n–p, 6a,b and 7b,c. Unprocessed blots are provided in the Source Data. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
Code availability
Code used in this study (such as JTK_CYCLE for rhythmic analysis) are referenced in the Methods sections above. Any other code used in the study can be obtained from the corresponding authors on reasonable request.
References
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010).
Dodd, A. N. et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (2005).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Reddy, A. B. & Rey, G. Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu. Rev. Biochem. 83, 165–189 (2014).
O’Neill, J. S. & Reddy, A. B. Circadian clocks in human red blood cells. Nature 469, 498–503 (2011).
Bass, J. & Takahashi, J. S. Circadian rhythms: redox redux. Nature 469, 476–478 (2011).
Sies, H., Berndt, C. & Jones, D. P. Oxidative stress. Annu. Rev. Biochem. 86, 715–748 (2017).
Winterbourn, C. C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 (2008).
Giorgio, M., Trinei, M., Migliaccio, E. & Pelicci, P. G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 8, 722–728 (2007).
D’Autreaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Yu, T. et al. In vitro and in vivo phase changes of the mouse circadian clock by oxidative stress. J. Circ. Rhythms 14, 4 (2016).
Putker, M. et al. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid. Redox Signal. 28, 507–520 (2017).
Wible, R. S. et al. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. eLife 7, e31656 (2018).
Ermakova, Y. G. et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 5, 5222 (2014).
Chang, H. C. & Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460 (2013).
Zhou, M., Diwu, Z., Panchuk-Voloshina, N. & Haugland, R. P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253, 162–168 (1997).
Ding, C. et al. Proteome-wide profiling of activated transcription factors with a concatenated tandem array of transcription factor response elements. Proc. Natl Acad. Sci. USA 110, 6771–6776 (2013).
Paulsen, C. E. & Carroll, K. S. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem. Rev. 113, 4633–4679 (2013).
Gupta, V. & Carroll, K. S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta 1840, 847–875 (2014).
Kim, J. R., Yoon, H. W., Kwon, K. S., Lee, S. R. & Rhee, S. G. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal. Biochem. 283, 214–221 (2000).
Gupta, V., Yang, J., Liebler, D. C. & Carroll, K. S. Diverse redoxome reactivity profiles of carbon nucleophiles. J. Am. Chem. Soc. 139, 5588–5595 (2017).
Klomsiri, C. et al. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol. 473, 77–94 (2010).
Fu, L. et al. Systematic and quantitative assessment of hydrogen peroxide reactivity with cysteines across human proteomes. Mol. Cell Proteom. 16, 1815–1828 (2017).
Martinez-Acedo, P., Gupta, V. & Carroll, K. S. Proteomic analysis of peptides tagged with dimedone and related probes. J. Mass Spectrom. 49, 257–265 (2014).
Huang, N. et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science 337, 189–194 (2012).
Stone, S. R., Hughes, M. J. & Jost, J. -P. in A laboratory Guide to in vitro Studies of Protein–DNA interactions (eds Saluz, H. P. & Becher, M. M.) 163–194 (Springer, 1991).
Asher, G. et al. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142, 943–953 (2010).
Landgraf, D., Wang, L. L., Diemer, T. & Welsh, D. K. NPAS2 compensates for loss of CLOCK in peripheral circadian oscillators. PLoS. Genet. 12, e1005882 (2016).
Esnault, C. et al. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 28, 943–958 (2014).
Migliaccio, E. et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 (1999).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 (2005).
Pinton, P. et al. Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315, 659–663 (2007).
Chen, Z. et al. Selective inhibition of protein kinase C β 2 attenuates the adaptor P66Shc-mediated intestinal ischemia-reperfusion injury. Cell Death Dis. 5, e1164 (2014).
Hunt, T. & Sassone-Corsi, P. Riding tandem: circadian clocks and the cell cycle. Cell 129, 461–464 (2007).
Koike, N. et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012).
Zhou, P., Ross, R. A., Pywell, C. M., Liangpunsakul, S. & Duffield, G. E. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci. Rep. 4, 3725 (2014).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
Sato, S. et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170, 664–677 (2017).
Conti, L. et al. Expression and activation of SH2/PTB-containing ShcA adaptor protein reflects the pattern of neurogenesis in the mammalian brain. Proc. Natl Acad. Sci. USA 94, 8185–8190 (1997).
Schibler, U. & Sassone-Corsi, P. A web of circadian pacemakers. Cell 111, 919–922 (2002).
Edgar, R. S. et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 485, 459–464 (2012).
Eckel-Mahan, K. L. et al. Reprogramming of the circadian clock by nutritional challenge. Cell 155, 1464–1478 (2013).
Solanas, G. et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170, 678–692 (2017).
Masri, S. et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158, 659–672 (2014).
Thaiss, C. A. et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510 (2016).
Masri, S. et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell 165, 896–909 (2016).
Zhao, X. et al. Circadian amplitude regulation via FBXW7-targeted REV-ERBα degradation. Cell 165, 1644–1657 (2016).
Solt, L. A. et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2012).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Ranieri, S. C. et al. Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA 107, 13420–13425 (2010).
Ciciliot, S. et al. p66Shc deletion or deficiency protects from obesity but not metabolic dysfunction in mice and humans. Diabetologia 58, 2352–2360 (2015).
Sone, K., Mori, M. & Mori, N. Selective upregulation of p66-Shc gene expression in the liver and brain of aged rats. Arch. Gerontol. Geriatr. 55, 744–748 (2012).
Jones, D. P. & Sies, H. The redox code. Antioxid. Redox Signal. 23, 734–746 (2015).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354, 994–999 (2016).
Wu, Y. et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 25, 73–85 (2017).
Mu, W. L. et al. Sox2 deacetylation by Sirt1 is involved in mouse somatic reprogramming. Stem Cells 33, 2135–2147 (2015).
Hu, Y. et al. ERK phosphorylates p66shcA on Ser36 and subsequently regulates p27kip1 expression via the Akt-FOXO3a pathway: implication of p27kip1 in cell response to oxidative stress. Mol. Biol. Cell 16, 3705–3718 (2005).
Zhou, S. et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 109, 639–648 (2011).
Kondratov, R. V. et al. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 17, 1921–1932 (2003).
Hida, A. et al. The human and mouse Period1 genes: five well-conserved E-boxes additively contribute to the enhancement of mPer1 transcription. Genomics 65, 224–233 (2000).
Shen, B. et al. Efficient genome modification by CRISPR–Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Huang, L. Z. et al. Whole-exome sequencing implicates UBE3D in age-related macular degeneration in east Asian populations. Nat. Commun. 6, 6687 (2015).
Rhee, S. G., Chang, T. S., Jeong, W. & Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 29, 539–549 (2010).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 (2007).
Walker, J. M. Nondenaturing polyacrylamide gel electrophoresis of proteins. Methods Mol. Biol. 32, 17–22 (1994).
Chen, H. Z. et al. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ. Res. 119, 1076–1088 (2016).
Lai, M. et al. Multidimensional proteomics reveals a role of UHRF2 in the regulation of epithelial–mesenchymal transition (EMT). Mol. Cell Proteom. 15, 2263–2278 (2016).
Savelyev, S. A., Larsson, K. C., Johansson, A. S. & Lundkvist, G. B. Slice preparation, organotypic tissue culturing and luciferase recording of clock gene activity in the suprachiasmatic nucleus. J. Vis. Exp. 15, 2439 (2011).
Acknowledgements
We thank X.-M. Xie and M. Lai (State Key Laboratory of Proteomics) for technical assistance and members of the De-Pei Liu laboratory for discussions about this project. This work was supported by grants from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS2017-I2M-1-008, 2019-RC-HL-006, 2016-I2M-1-015 and 2016-I2M-1-011), the National Natural Science Foundation of China (nos. 91849207, 81701387, 91639304, 31471126 and 31571193), the Medical Epigenetics Research Center, Chinese Academy of Medical Sciences (2017PT31035 and 2018PT31015) and Special Financial Grant from the China Postdoctoral Science Foundation (2017T100051). H.-Z.C. is also supported by the Youth Top-notch Talent Support Program and the Youth Yangtze River Scholar Program in China.
Author information
Authors and Affiliations
Contributions
J.-F.P., H.-Z.C. and D.-P.L. conceived the project. J.-F.P., Q.G., Y.Z., X.-K.L. and W.-Q.L. performed mouse locomotor experiments and tissue collection. J.-F.P., X.-K.L., W.-Q.L., Y.Z., J.-Q.F. and S.-S.C. performed H2O2 assays, reporter assays, DCP-Bio1, BTD and BIAM labelling. Y.Z. and X.-K.L. constructed vectors and site-directed mutagenesis. X.-K.L. purified proteins and performed experiments about knockin mice. X.-K.L., W.-Q.L. and J.-F.P. performed EMSA and non-denaturing PAGE. J.-F.P. and X.-M.W analysed RNA-seq data. Y.Z., X.Z. and D.-L.H. performed Clock knockout mice experiments. J.Y. performed mass spectrometry and provided intellectual support for redox subject. J.Y. and K.S.C. provided technical support for redox modification examination. J.-F.P. performed real-time luciferase assays with help from D.J. and N.L. E.E.Z. conceived the LumiCycle design and provided intellectual support for the project. J.-F.P. prepared the illustrations and wrote the manuscript under the guidance of H.-Z.C. and D.-P.L. J.-H.Q. and J.-M.C. contributed to revision of characters. All of the authors contributed to data analysis and reviewed the manuscript. H.-Z.C. and D.-P.L. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Endogenous H2O2 levels oscillate rhythmically in cells.
a, Time-lapse microscopy of circadian HyPerRed fluorescence in three individual cells for one day post-serum shock. N2a cells were transfected with HyPerRed, and images were obtained every 30 min (n = 3 independent experiments with similar results). b, Time-lapse microscopy of circadian HyPerRed fluorescence in three individual cells for one day without a serum treatment. U2OS cells were stably expressing HyPerRed, and images were obtained every 30 min. Note that the three cells have widely different phases (n = 3 independent experiments with similar results). c, Time-lapse microscopy of HyPerRed and GFP fluorescence under the same promoter in the same cell for one day without a serum treatment. U2OS cells were stably expressing HyPerRed and GFP, and images were obtained every 30 min (n = 3 independent experiments with similar results). Source data are provided in Statistics Source Data Extended Data Fig. 1.
Extended Data Fig. 2 H2O2-sensitive transcription factor (TF) screening identifies that the redox state of CLOCK oscillates rhythmically.
a, Reactive thiols of cysteine residues in proteins are oxidized to S-sulfenic acids (–SOH) by H2O2. Sulfenic acids are unstable and further react with proximal thiol groups to form disulfides, which are reversible oxidative modifications that can be restored to free thiols. b, Changes in the redox state of cysteine residues in proteins are monitored by detecting free thiols with biotin-conjugated iodoacetamide (BIAM) and by detecting cysteine sulfenic acid (R-SOH) with DCP-Bio1 or BTD. c, Representative western blot of S-sulfenylated CLOCK (CLOCK-SOH) from mouse livers labelled by DCP-Bio1. Biotin-blocked streptavidin-magnetic beads were used as a negative control. d, Representative western blot of free reactive thiols in CLOCK labelled by BIAM in vivo and in vitro from N2a cells. Biotin-blocked streptavidin-magnetic beads were used as a negative control. e, Representative western blot of free thiols of CLOCK labelled by BIAM from N2a cells treated with H2O2 (200 μM) for 0, 5, or 20 min and fractionated, followed by immunoprecipitation with CLOCK antibody. f, Relative levels of CCG transcripts in MEFs treated with H2O2 (200 μM) for 12 h. Data are presented as the means ± SEM (n = 3 independent biological samples). P values were calculated using an unpaired two-tailed Student’s t test. g, Relative HyPerRed intensity in U2OS cells stably expressing HyPerRed throughout the circadian cycle (n = 6 independent biological samples). h, Representative western blot of CLOCK-SOH labelled by DCP-Bio1 in U2OS cells over the circadian cycle. n = 3 independent experiments for c,d and n = 2 independent experiments for e,h with similar results. Source data are provided in Statistics Source Data Extended Data Fig. 2. Unprocessed blots are shown in Source Data Extended Data Fig. 2.
Extended Data Fig. 3 Cysteine195 mediates the rhythmic oscillations of CLOCK’s redox state.
a, Expression levels of each mutant CLOCK plasmid in HEK293T cells examined by immunoblotting (n = 2 independent experiments with similar results). b, Relative luciferase activities of Per1:Luc in HEK293T cells transfected with WT or C195S mutant CLOCK plasmids in the absence or presence of BMAL1 (n = 3 independent biological samples). Data are presented as the means ± SEM. c, Schematic of the target site at the Clock locus. In the double-stranded DNA, the sgRNA target is shown in blue and the PAM sequence is shown in red. Red arrowhead indicates the Cas9 cleavage site. In the donor DNA, the replaced nucleotides are shown in red (for point mutation) or blue (for silent mutation). d,e, Representative western blot (d) and quantification (e) of CLOCK-SOH labelled by DCP-Bio1 in MAFs from WT and ClockC195S mice for one circadian cycle (n = 2 independent experiments with similar results). Source data are provided in Statistics Source Data Extended Data Fig. 3. Unprocessed blots are shown in Source Data Extended Data Fig. 3.
Extended Data Fig. 4 Redox regulation of CLOCK at Cysteine195 is essential for normal clock function.
a, Schematic of the recombinant WT/C195S-CLOCK (amino acids 26–384) and BMAL1 (amino acids 62–447) proteins. b, Coomassie Brilliant Blue staining of the recombinant BMAL1 and WT/C195S-CLOCK proteins. c,d, Representative image of a non-denaturing polyacrylamide gel electrophoresis (PAGE) gel showing the heterodimer of recombinant BMAL1 and WT- or C195S-CLOCK treated with or without different concentrations of H2O2. e, Representative image of a non-denaturing PAGE gel of the heterodimer of the recombinant BMAL1 protein and increasing concentrations of the recombinant WT-CLOCK protein. f,g, Representative EMSA of recombinant BMAL1 or the heterodimer of recombinant BMAL1 and WT-CLOCK binding to increasing concentrations of the G-box probe (f) and the heterodimer binding to G-box probe treated with or without H2O2 (10−5 mM) (g). h, Relative mRNA levels of Bmal1 in WT and ClockC195S MAFs over the circadian cycles (n = 3 independent biological samples per time point). i, Schematic of 20-nt sgRNA target sequence of Clock (blue) and PAM (red). Red arrowhead indicates Cas9 cleavage site. j, Sequencing of PCR product from a Clock KO mouse. Black arrows indicate the location of mutations introduced by CRISPR/Cas9. k, Representative immunoblot of CLOCK in WT and Clock KO MAFs. l, ChIP analysis of WT- and C195S-CLOCK proteins binding to the Dbp promoter (-1360 to -1297) (-508 to -414) in N2a cells. The Rpl19 promoter served as the negative control (n = 3 independent biological samples). P values were calculated using one way ANOVA with a Bonferroni’s post hoc test. n = 2 independent experiments for b-g,j,k with similar results. Data are presented as the means ± SEM. Source data are provided in Statistics Source Data Extended Data Fig. 4. Unprocessed blots are shown in Source Data Extended Data Fig. 4.
Extended Data Fig. 5 P66Shc is indispensable for robust oscillations of H2O2 levels and normal CLOCK function.
a, Relative H2O2 concentration in N2a cells transfected with a gradient mass of p66Shc (n = 3 independent biological samples). b, Representative immunoblot of p66Shc phosphorylation at Ser36 in MEFs treated with LY333531 (1 μM). c-i, Relative mRNA levels or representative immunoblot of p66Shc in MEFs (c,d), in livers (e,f), in N2a cells overexpressing CLOCK and BMAL1 (g), or in WT MAFs and Cry DKO MAFs (h,i) (n = 3 independent biological samples per group for c,e,g,h). j,k, Analysis of E-box elements on the promoter of mouse p66Shc (5 kb) (j) and its evolutionary conservation among multiple species (k). l, Representative immunoblot of reduced HA-CLOCK after transient transfection of p66Shc. m, Representative immunoblot of p66Shc in p66Shc KO MEFs rescued by p66Shc overexpression. n, Relative mRNA levels of CCGs in N2a cells overexpressing the WT or S36A mutant of p66Shc (n = 4 independent biological samples, except the Nampt in p66Shc group where n = 5, and the Per2 in p66Shc group and the Wee1 in p66S36A group where n = 3). o, Relative levels of Nampt mRNA in N2a cells overexpressing WT or S36A mutant of p66Shc and treated with H2O2 (200 μM) or catalase (1000 U/ml) (n = 5 independent biological samples). p, Relative amplitude of mPER2::LUC bioluminescence rhythms from WT and p66Shc KO liver explants (n = 9 independent biological samples). P values are shown for the comparisons to the control (a,g), to the first time point for p66Shc (c,e), to Cry DKO (h), to p66Shc (n), to p66S36A (o), and to WT group (p). P values were calculated using an unpaired two-tailed Student’s t test (n,p) and one-way ANOVA with a Bonferroni’s post hoc test (a,c,e,g,h,o). Data are presented as the means ± SEM. n = 3 independent experiments for b,d,f,i,m and n = 2 independent experiments for l with similar results. Source data are provided in Statistics Source Data Extended Data Fig. 5. Unprocessed blots are shown in Source Data Extended Data Fig. 5.
Extended Data Fig. 6 P66Shc KO reprograms hepatic transcriptome oscillations.
a, Phase analysis of transcripts that oscillate only in the WT or p66Shc KO group. b, Phase analysis of genes that retain oscillations in both WT and p66Shc KO mice. RNA-seq analysis of the whole transcriptome was performed using total RNA obtained from three mouse livers each time point, which were pooled and then divided into two samples, at 4-h intervals for one circadian cycle under DD conditions. All analyses are from n = 1 RNA-sequencing experiment. Source data are provided in Statistics Source Data Extended Data Fig. 6.
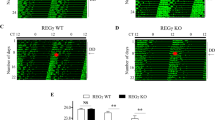
Extended Data Fig. 7 P66Shc modulates circadian behaviours in mice.
a, Representative western blot of p66Shc in mouse SCN and cortex at CT8 and CT14 under DD conditions. Three mouse SCNs or cortices were pooled per time point (n = 3 independent experiments with similar results). b, Relative levels of p66Shc mRNA in the SCN for one circadian cycle under DD conditions (n = 3 independent biological samples per time point). P values are shown for the comparisons to the first time point for p66Shc. Data are presented as the means ± SEM. c, Representative double-plot actograms of wheel-running activities and period lengths from two WT (left) and two p66Shc KO female mice (right) under DD conditions after LD entrainment. Red lines indicate the day on which DD conditions were initiated. P values are shown for the comparisons of p66Shc KO with WT. Values are presented as the means ± SEM (n = 9 mice). P values were calculated using one-way ANOVA with a Bonferroni’s post hoc test (b) and an unpaired two-tailed Student’s t test (c). Source data are provided in Statistics Source Data Extended Data Fig. 7. Unprocessed blots are shown in Source Data Extended Data Fig. 7.
Supplementary information
Supplementary Table
Supplementary Table 1. TFs identified in control and H2O2-treated groups. Supplementary Table 2. Information of nucleotide sequences used in the study. Supplementary Table 3. RNA-seq results in WT and p66Shc KO mouse livers for one circadian cycle.
Source data
Source Data Fig. 1
Statistical Source Data Figure 1
Source Data Fig. 2
Statistical Source Data Figure 2
Source Data Fig. 2
Unprocessed Western Blots and/or gels for Figure 2
Source Data Fig. 3
Statistical Source Data Figure 3
Source Data Fig. 3
Unprocessed Western Blots and/or gels for Figure 3
Source Data Fig. 4
Statistical Source Data Figure 4
Source Data Fig. 4
Unprocessed Western Blots and/or gels for Figure 4
Source Data Fig. 5
Statistical Source Data Figure 5
Source Data Fig. 5
Unprocessed Western Blots and/or gels for Figure 5
Source Data Fig. 6
Statistical Source Data Figure 6
Source Data Fig. 7
Statistical Source Data Figure 7
Data Extended Data Fig. 1
Statistics Source Data Extended Data Figure 1
Data Extended Data Fig. 2
Statistics Source Data Extended Data Figure 2
Data Extended Data Fig. 2
Unprocessed Western Blots and/or gels for Extended Data Figure 2
Data Extended Data Fig. 3
Statistics Source Data Extended Data Figure 3
Data Extended Data Fig. 3
Unprocessed Western Blots and/or gels for Extended Data Figure 3
Data Extended Data Fig. 4
Statistics Source Data Extended Data Figure 4
Data Extended Data Fig. 4
Unprocessed Western Blots and/or gels for Extended Data Figure 4
Data Extended Data Fig. 5
Statistics Source Data Extended Data Figure 5
Data Extended Data Fig. 5
Unprocessed Western Blots and/or gels for Extended Data Figure 5
Data Extended Data Fig. 6
Statistics Source Data Extended Data Figure 6
Data Extended Data Fig. 7
Statistics Source Data Extended Data Figure 7
Data Extended Data Fig. 7
Unprocessed Western Blots and/or gels for Extended Data Figure 7
Rights and permissions
About this article
Cite this article
Pei, JF., Li, XK., Li, WQ. et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat Cell Biol 21, 1553–1564 (2019). https://doi.org/10.1038/s41556-019-0420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0420-4
This article is cited by
-
Circadian clock disruption promotes the degeneration of dopaminergic neurons in male Drosophila
Nature Communications (2023)
-
Oxidized galectin-1 in SLE fails to bind the inhibitory receptor VSTM1 and increases reactive oxygen species levels in neutrophils
Cellular & Molecular Immunology (2023)
-
Dynamic monitoring of oscillatory enzyme activity of individual live bacteria via nanoplasmonic optical antennas
Nature Photonics (2023)
-
A puromycin-dependent activity-based sensing probe for histochemical staining of hydrogen peroxide in cells and animal tissues
Nature Protocols (2022)
-
Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology
Nature Reviews Molecular Cell Biology (2022)