Abstract
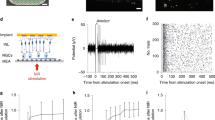
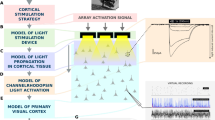
Retinal prostheses can restore a functional form of vision in patients affected by dystrophies of the outer retinal layer. Beyond clinical utility, prostheses for the stimulation of the optic nerve, the visual thalamus or the visual cortex could also serve as tools for studying the visual system. Optic-nerve stimulation is particularly promising because it directly activates nerve fibres, takes advantage of the high-level information processing occurring downstream in the visual pathway, does not require optical transparency and could be effective in cases of eye trauma. Here we show, in anaesthetized rabbits and with support from numerical modelling, that an intraneural electrode array with high mechanical stability placed in the intracranial segment of the optic nerve induces, on electrical stimulation, selective activation patterns in the visual cortex. These patterns are measured as electrically evoked cortical potentials via an ECoG array placed in the contralateral cortex. The intraneural electrode array should enable further investigations of the effects of electrical stimulation in the visual system and could be further developed as a visual prosthesis for blind patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that all data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request.
Code availability
Code used for the hybrid FEA model and NEURON simulation is available at https://github.com/lne-lab/nBME2019. The authors declare that the algorithm used for blind source separation is described in the referenced papers. The source code can be obtained from S.M. (silvestro.micera@epfl.ch) upon reasonable request.
References
Bourne, R. R. et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob. Health 1, e339–e349 (2013).
Brindley, G. & Lewin, W. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 196, 479–493 (1968).
Dobelle, W., Mladejovsky, M. & Girvin, J. Artificial vision for the blind: Electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 183, 440–444 (1974).
Zrenner, E. Fighting blindness with microelectronics. Sci. Transl. Med. 5, 210ps16 (2013).
Ghezzi, D. Retinal prostheses: progress toward the next generation implants. Front. Neurosci. 9, 290 (2015).
Stingl, K. et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. R. Soc. B 280, 20130077 (2013).
da Cruz, L. et al. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254 (2016).
Ayton, L. N. et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE 9, e115239 (2014).
Brelén, M. E., Vince, V., Gérard, B., Veraart, C. & Delbeke, J. Measurement of evoked potentials after electrical stimulation of the human optic nerve. Invest. Ophthalmol. Vis. Sci. 51, 5351–5355 (2010).
Panetsos, F., Sanchez-Jimenez, A., Cerio, E., Diaz-Guemes, I. & Sanchez, F. M. Consistent phosphenes generated by electrical microstimulation of the visual thalamus. An experimental approach for thalamic visual neuroprostheses. Front. Neurosci. 5, 84 (2011).
Normann, R. A. et al. Toward the development of a cortically based visual neuroprosthesis. J. Neural Eng. 6, 035001 (2009).
Merabet, L. B., Rizzo, J. F., Amedi, A., Somers, D. C. & Pascual-Leone, A. What blindness can tell us about seeing again: merging neuroplasticity and neuroprostheses. Nat. Rev. Neurosci. 6, 71–77 (2005).
Luo, Y. & da Cruz, L. The Argus II retinal prosthesis system. Prog. Retin. Eye Res. 50, 89–107 (2016).
Stingl, K. et al. Subretinal visual implant Alpha IMS—Clinical trial interim report. Vis. Res. 111, 149–160 (2015).
Lorach, H. et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 21, 476–482 (2015).
Ferlauto, L. et al. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 9, 992 (2018).
Antognazza, M. et al. Shedding light on living cells. Adv. Mater. 27, 7662–7669 (2015).
Maya-Vetencourt, J. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 16, 681–689 (2017).
Antognazza, M. et al. Characterization of a polymer‐based, fully organic prosthesis for implantation into the subretinal space of the rat. Adv. Health. Mater. 5, 2271–2282 (2016).
Veraart, C. et al. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res. 813, 181–186 (1998).
Brelén, M. E. et al. Intraorbital implantation of a stimulating electrode for an optic nerve visual prosthesis. J. Neurosur. 104, 593–597 (2006).
Duret, F. et al. Object localization, discrimination, and grasping with the optic nerve visual prosthesis. Restor. Neurol. Neurosci. 24, 31–40 (2006).
Veraart, C., Wanet‐Defalque, M., Gérard, B., Vanlierde, A. & Delbeke, J. Pattern recognition with the optic nerve visual prosthesis. Artif. Organs 27, 996–1004 (2003).
Brelén, M., Duret, F., Gérard, B., Delbeke, J. & Veraart, C. Creating a meaningful visual perception in blind volunteers by optic nerve stimulation. J. Neural Eng. 2, S22 (2005).
The Lasker/IRRF Initiative for Innovation in Vision Science. Chapter 1 - Restoring vision to the blind: The new age of implanted visual prostheses. Transl. Vis. Sci. Technol. https://doi.org/10.1167/tvst.3.7.3 (2014).
Yan, Y. et al. Electrically evoked responses in the rabbit cortex induced by current steering with penetrating optic nerve electrodes. Invest. Ophthalmol. Vis. Sci. 57, 6327–6338 (2016).
Sun, J., Chen, Y., Chai, X., Ren, Q. & Li, L. Penetrating electrode stimulation of the rabbit optic nerve: parameters and effects on evoked cortical potentials. Graefes Arch. Clin. Exp. Ophthalmol. 251, 2545–2554 (2013).
Lu, Y. et al. Electrical stimulation with a penetrating optic nerve electrode array elicits visuotopic cortical responses in cats. J. Neural Eng. 10, 036022 (2013).
Raspopovic, S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19–222ra19 (2014).
Oddo, C. et al. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 5, e09148 (2016).
Raspopovic, S., Capogrosso, M., Badia, J., Navarro, X. & Micera, S. Experimental validation of a hybrid computational model for selective stimulation using transverse intrafascicular multichannel electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 20, 395–404 (2012).
Badia, J. et al. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. J. Neural Eng. 8, 036023 (2011).
Cutrone, A. et al. A three-dimensional self-opening intraneural peripheral interface (SELINE). J. Neural Eng. 12, 016016 (2015).
Wurth, S. et al. Long-term usability and bio-integration of polyimide-based intra-neural stimulating electrodes. Biomaterials 122, 114–129 (2017).
Hukins, D. W. L., Mahomed, A. & Kukureka, S. N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 30, 1270–1274 (2008).
Howlader, M., Doyle, T., Mohtashami, S. & Kish, J. Charge transfer and stability of implantable electrodes on flexible substrate. Sens. Actuators B Chem. 178, 132–139 (2013).
Giolli, R. & Guthrie The primary optic projections in the rabbit. An experimental degeneration study. J. Com. Neurol. 136, 99–126 (1969).
Sun, J. et al. Spatiotemporal properties of multipeaked electrically evoked potentials elicited by penetrative optic nerve stimulation in rabbits. Invest. Ophthalmol. Vis. Sci. 52, 146–154 (2011).
Delorme, A., Palmer, J., Onton, J., Oostenveld, R. & Makeig, S. Independent EEG sources are dipolar. PLoS ONE 7, e30135 (2012).
Menicucci, D. et al. Brain responses to emotional stimuli during breath holding and hypoxia: an approach based on the independent component analysis. Brain Topogr. 27, 771–785 (2014).
Artoni, F. et al. Unidirectional brain to muscle connectivity reveals motor cortex control of leg muscles during stereotyped walking. NeuroImage 159, 403–416 (2017).
Bell, A. & Sejnowski, T. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 7, 1129–1159 (1995).
Delbeke, J., Oozeer, M. & Veraart, C. Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vis. Res. 43, 1091–1102 (2003).
Li, M. et al. A simulation of current focusing and steering with penetrating optic nerve electrodes. J. Neural Eng. 10, 066007 (2013).
Ghezzi, D. et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 7, 400–406 (2013).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 6, 391–397 (2012).
Mandel, Y. et al. Cortical responses elicited by photovoltaic subretinal prostheses exhibit similarities to visually evoked potentials. Nat. Commun. 4, 1980 (2013).
Tang, J. et al. Nanowire arrays restore vision in blind mice. Nat. Commun. 9, 786 (2018).
Sakaguchi, H. et al. Artificial vision by direct optic nerve electrode (AV-DONE) implantation in a blind patient with retinitis pigmentosa. J. Artif. Organs 12, 206–209 (2009).
Boinagrov, D., Pangratz-Fuehrer, S., Goetz, G. & Palanker, D. Selectivity of direct and network-mediated stimulation of the retinal ganglion cells with epi-, sub- and intraretinal electrodes. J. Neural Eng. 11, 026008 (2014).
Weiland, J. D., Walston, S. T. & Humayun, M. S. Electrical stimulation of the retina to produce artificial vision. Annu. Rev. Vis. Sci. 2, 273–294 (2016).
Nirenberg, S. & Pandarinath, C. Retinal prosthetic strategy with the capacity to restore normal vision. Proc. Natl Acad. Sci. USA 109, 15012–15017 (2012).
Piedade, M., Gerald, J., Sousa, L., Tavares, G. & Tomás, P. Visual neuroprosthesis: a non invasive system for stimulating the cortex. IEEE Trans. Circuits Syst. I 52, 2648–2662 (2005).
Sieu, L.-A. A. et al. EEG and functional ultrasound imaging in mobile rats. Nat. Methods 12, 831–834 (2015).
Demene, C. et al. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 9, eaah6756 (2017).
Blaize, K. et al. Functional ultrasound imaging of deep visual cortex in awake non-human primates. Preprint at Biorxiv https://doi.org/10.1101/551663 (2019).
Palmer, J. A., Kreutz-Delgado, K., Rao, B. D. & Makeig, S. in Independent Component Analysis and Signal Separation (eds Davies M.E. et al.) 90–97 (Springer, 2007).
Artoni, F., Menicucci, D., Delorme, A., Makeig, S. & Micera, S. RELICA: a method for estimating the reliability of independent components. NeuroImage 103, 391–400 (2014).
Artoni, F., Delorme, A. & Makeig, S. Applying dimension reduction to EEG data by principal component analysis reduces the quality of its subsequent independent component decomposition. NeuroImage 175, 176–187 (2018).
Goodall, E. V., Kosterman, L. M., Holsheimer, J. & Struijk, J. J. Modeling study of activation and propagation delays during stimulation of peripheral nerve fibers with a tripolar cuff electrode. IEEE Trans. Rehabil. Eng. 3, 272–282 (1995).
Chintalacharuvu, R. R., Ksienski, D. A. & Mortimer, J. T. A numerical analysis of the electric field generated by a nerve cuff electrode. Proc. Annual International Conference of the IEEE Engineering in Medicine and Biology Society 912-913 (IEEE, 1991).
Struijk, J. J., Holsheimer, J., Barolat, G., He, J. & Boom, H. B. K. Paresthesia thresholds in spinal cord stimulation: a comparison of theoretical results with clinical data. IEEE Trans. Rehabil. Eng. 1, 101–108 (1993).
Bossetti, C. A., Birdno, M. J. & Grill, W. M. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J. Neural Eng. 5, 44 (2008).
McIntyre, C. C. & Grill, W. M. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 88, 1592–1604 (2002).
Raspopovic, S., Capogrosso, M. & Micera, S. A computational model for the stimulation of rat sciatic nerve using a transverse intrafascicular multichannel electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 333–344 (2011).
Hodgkin, A. & Huxley, A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Oozeer, M., Veraart, C., Legat, V. & Delbeke, J. A model of the mammalian optic nerve fibre based on experimental data. Vis. Res. 46, 2513–2524 (2006).
Acknowledgements
We acknowledge the support from the Bioelectron Microscopy Core Facility (BIOEM) of École polytechnique fédérale de Lausanne. This work has been supported by École polytechnique fédérale de Lausanne, Medtronic, Bertarelli Foundation and Wyss Center for Bio and Neuroengineering. F.A. is supported by the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie Action agreement no. 750947 (BIREHAB).
Author information
Authors and Affiliations
Contributions
V.G. designed the stimulation protocol and performed the modelling and simulation, blind source separation and data analysis. A.C. designed and fabricated the OpticSELINE and performed mechanical and electrochemical characterizations. F.A. conceived and performed the blind source separation approach. P.V. performed in vivo and histological experiments. A.M.P. performed the modelling and simulation. S.A.R. participated in the design of the stimulation protocol and data analysis. D.L.D.P. participated in the design and microfabrication of the OpticSELINE and performed mechanical characterizations. S.M. supervised the activities related to electrode development and the blind source separation approach. D.G. designed the study, led the project and wrote the manuscript. All the authors read, edited, and accepted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Gaillet, V., Cutrone, A., Artoni, F. et al. Spatially selective activation of the visual cortex via intraneural stimulation of the optic nerve. Nat Biomed Eng 4, 181–194 (2020). https://doi.org/10.1038/s41551-019-0446-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0446-8
This article is cited by
-
An actor-model framework for visual sensory encoding
Nature Communications (2024)
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
Cortico-muscular connectivity is modulated by passive and active Lokomat-assisted Gait
Scientific Reports (2023)
-
Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities
Nature Biomedical Engineering (2022)
-
Photovoltaic retinal prosthesis restores high-resolution responses to single-pixel stimulation in blind retinas
Communications Materials (2021)