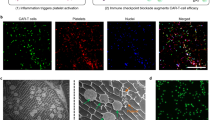
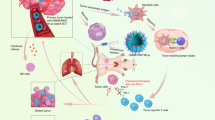
Abstract
Radiation therapy for cancer can lead to off-target toxicity and can be ineffective against hypoxic solid tumours and distant metastases. Here, we show that intratumoral injection, in mouse and rabbit xenografts and in patient-derived mouse xenografts, of a sodium alginate formulation containing catalase (Cat) labelled with the therapeutic 131I radioisotope enables long-term relief of tumour hypoxia and complete tumour elimination at low radioactivity doses. On injection, the soluble polysaccharide rapidly transforms into a hydrogel in the presence of endogenous Ca2+, fixing 131I-Cat within the tumours. We also show that local radiotherapy with a formulation that includes the immunostimulatory CpG oligonucleotide combined with systemic checkpoint-blockade therapy using an anti-CTLA-4 antibody leads to metastasis inhibition and protection against tumour rechallenge. The local therapy, which uses only biocompatible components, might enable new strategies for local tumour treatments that can be combined with systemic therapeutic responses, for the inhibition of tumour metastasis and the prevention of tumour recurrence in patients with advanced-stage cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kjellberg, R. N. Radiation therapy. Science 176, 1071 (1972).
Delaney, G., Jacob, S., Featherstone, C. & Barton, M. The role of radiotherapy in cancer treatment. Cancer 104, 1129–1137 (2005).
Baskar, R., Lee, K. A., Yeo, R. & Yeoh, K.-W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9, 193–199 (2012).
Bristow, R. G. & Hill, R. P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8, 180–192 (2008).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Kim, J. & Jung, Y. Radiation-induced liver disease: current understanding and future perspectives. Exp. Mol. Med. 49, e359 (2017).
Dietrich, G. Radiation injuries caused by radium and radioisotope therapy. Strahlentherapie 114, 128–134 (1961).
Hilaris, B. S., Henschke, U. K. & Holt, J. G. Clinical experience with long half-life and low-energy encapsulated radioactive sources in cancer radiation therapy. Radiology 91, 1163–1167 (1968).
Zhu, H.-D. et al. Conventional stents versus stents loaded with 125iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol. 15, 612–619 (2014).
D’Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J. Am. Med. Assoc. 280, 969–974 (1998).
Nath, R. et al. Dosimetry of interstitial brachytherapy sources—recommendations of the AAPM Radiation-Therapy Committee Task Group No 43. Med. Phys. 22, 209–234 (1995).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. 58, 862–870 (2004).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Peppas, N. A., Bures, P., Leobandung, W. & Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Nucl. Med. Mol. 50, 27–46 (2000).
Gisby, P. E. & Hall, D. O. Biophotolytic H2 production using alginate-immobilized chloroplasts, enzymes and synthetic catalysts. Nature 287, 251–253 (1980).
Hayashi, K., Sakamoto, W. & Yogo, T. Smart ferrofluid with quick gel transformation in tumors for MRI-guided local magnetic thermochemotherapy. Adv. Funct. Mater. 26, 1708–1718 (2016).
Song, G. et al. Catalase-loaded TaOx nanoshells as bio-nanoreactors combining high-Z element and enzyme delivery for enhancing radiotherapy. Adv. Mater. 28, 7143–7148 (2016).
Gungor, B. et al. CpG ODN nanorings induce IFNα from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci. Transl. Med. 6, 235ra261 (2014).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535, 65–74 (2016).
Berson, S. A. & Yalow, R. S. Iodoinsulin used to determine specific activity of iodine-131. Science 152, 205–207 (1966).
Larson, S. M., Carrasquillo, J. A., Cheung, N.-K. V. & Press, O. W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 15, 347–360 (2015).
Chen, Q. et al. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J. Control. Release 263, 79–89 (2017).
Zhang, Y., Hong, H. & Cai, W. Photoacoustic imaging. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.top065508 (2011).
Julien, S. et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 18, 5314–5328 (2012).
Inoue, T., Terada, N., Kobayashi, T. & Ogawa, O. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat. Rev. Urol. 14, 267–283 (2017).
Zhong, H. et al. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res. 59, 5830–5835 (1999).
Herskovic, A. et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 326, 1593–1598 (1992).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Tang, C. et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol. Res. 2, 831–838 (2014).
Rodriguez-Ruiz, M. E. et al. Brachytherapy attains abscopal effects when combined with immunostimulatory monoclonal antibodies. Brachytherapy 16, 1246–1251 (2017).
Wang, C. et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 26, 8154–8162 (2014).
Chen, Q. et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016).
Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5, 471–484 (2006).
Pavet, V., Portal, M. M., Moulin, J. C., Herbrecht, R. & Gronemeyer, H. Towards novel paradigms for cancer therapy. Oncogene 30, 1–20 (2011).
Keu, K. V. et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med. 9, aag2196 (2017).
Quezada, S. A., Peggs, K. S., Curran, M. A. & Allison, J. P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945 (2006).
Zaidi, M. R. & Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 17, 6118–6124 (2011).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Nag, S., Beyer, D., Friedland, J., Grimm, P. & Nath, R. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int. J. Radiat. Oncol. 44, 789–799 (1999).
Heemskerk, B., Kvistborg, P. & Schumacher, T. N. M. The cancer antigenome. EMBO J. 32, 194–203 (2013).
Xing, R. et al. An injectable self-assembling collagen–gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 28, 3669–3676 (2016).
Hussain, A. A., Jona, J. A., Yamada, A. & Dittert, L. W. Chloramine-T in radiolabeling techniques. 2. A nondestructive method for radiolabeling biomolecules by halogenation. Anal. Biochem. 224, 221–226 (1995).
Tian, L. et al. Radionuclide I-131 labeled albumin-paclitaxel nanoparticles for synergistic combined chemo-radioisotope therapy of cancer. Theranostics 7, 614–623 (2017).
Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 196, 143–151 (1991).
Chao, Y. et al. Dataset for ‘Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses’. figshare https://doi.org/10.6084/m9.figshare.6292136 (2018).
Acknowledgements
This work was partially supported by the National Basic Research Programs of China (973 Program) (2016YFA0201200), the National Natural Science Foundation of China (51525203, 51761145041, 81471716 and 31400861), the Collaborative Innovation Center of Suzhou Nano Science and Technology, a ‘111’ program from the Ministry of Education of China, and a Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Y.C., K.Y. and Z.L. designed the project. Y.C., L.X., C.L., L.F., J.X., Z.D., L.T. and X.Y. performed the experiments. Y.C., K.Y. and Z.L. analysed and interpreted the data. Z.L. supervised the overall research. Y.C. and Z.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Chao, Y., Xu, L., Liang, C. et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng 2, 611–621 (2018). https://doi.org/10.1038/s41551-018-0262-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0262-6
This article is cited by
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)
-
Biomaterials tools to modulate the tumour microenvironment in immunotherapy
Nature Reviews Bioengineering (2023)
-
A photo-triggered self-accelerated nanoplatform for multifunctional image-guided combination cancer immunotherapy
Nature Communications (2023)
-
Aerosolized immunotherapeutic nanoparticle inhalation potentiates PD-L1 blockade for locally advanced lung cancer
Nano Research (2023)
-
Immunotherapy for lung cancer combining the oligodeoxynucleotides of TLR9 agonist and TGF-β2 inhibitor
Cancer Immunology, Immunotherapy (2023)