Abstract
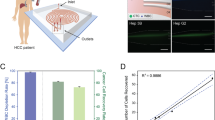
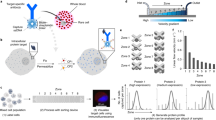
The detection and analysis of rare blood biomarkers is necessary for early diagnosis of cancer and to facilitate the development of tailored therapies. However, current methods for the isolation of circulating tumour cells (CTCs) or nucleic acids present in a standard clinical sample of only 5–10 ml of blood provide inadequate yields for early cancer detection and comprehensive molecular profiling. Here, we report the development of a flexible magnetic wire that can retrieve rare biomarkers from the subject’s blood in vivo at a much higher yield. The wire is inserted and removed through a standard intravenous catheter and captures biomarkers that have been previously labelled with injected magnetic particles. In a proof-of-concept experiment in a live porcine model, we demonstrate the in vivo labelling and single-pass capture of viable model CTCs in less than 10 s. The wire achieves capture efficiencies that correspond to enrichments of 10–80 times the amount of CTCs in a 5-ml blood draw, and 500–5,000 times the enrichments achieved using the commercially available Gilupi CellCollector.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 December 2019
In the version of this Article originally published, the ORCID for Sanjiv S. Gambhir was incorrect; the correct ORCID is 0000-0002-2711-7554. This has now been amended.
References
Yu, M., Stott, S., Toner, M., Maheswaran, S. & Haber, D. A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 192, 373–382 (2011).
Coumans, F. A. W., Ligthart, S. T., Uhr, J. W. & Terstappen, L. W. M. M. Challenges in the enumeration and phenotyping of CTC. Clin. Cancer Res. 18, 5711–5718 (2012).
Alix-Panabières, C. & Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14, 623–631 (2014).
Yu, M. et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Cayrefourcq, L. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 892–901 (2015).
Stoecklein, N. H., Fischer, J. C., Niederacher, D. & Terstappen, L. W. M. M. Challenges for CTC-based liquid biopsies: low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev. Mol. Diagn. 16, 147–164 (2016).
Adams, J. D., Kim, U. & Soh, H. T. Multitarget magnetic activated cell sorter. Proc. Natl Acad. Sci. USA 105, 18165–18170 (2008).
Earhart, C. M. et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip 14, 78–88 (2014).
Kang, J. H. et al. A combined micromagnetic–microfluidic device for rapid capture and culture of rare circulating tumor cells. Lab Chip 12, 2175 (2012).
Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 7, 1644 (2007).
Inglis, D. W., Riehn, R., Sturm, J. C. & Austin, R. H. Microfluidic high gradient magnetic cell separation. J. Appl. Phys. 99, 08K101 (2006).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl Med. 5, 179ra47 (2013).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Che, J. et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 7, 12748–12760 (2016).
Chung, J. et al. Rare cell isolation and profiling on a hybrid magnetic/size-sorting chip. Biomicrofluidics 7, 54107 (2013).
Hou, S. et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Adv. Mater. 25, 1547–1551 (2013).
Fischer, J. C. et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc. Natl Acad. Sci. USA 110, 16580–16585 (2013).
Herrmann, I. K. et al. Device for continuous extracorporeal blood purification using target-specific metal nanomagnets. Nephrol. Dial. Transplant. 26, 2948–2954 (2011).
Saucedo-Zeni, N. et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 41, 1241–1250 (2012).
Laurent, S., Saei, A. A., Behzadi, S., Panahifar, A. & Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin. Drug Deliv. 11, 1449–1470 (2014).
Fu, A. et al. Fluorescent magnetic nanoparticles for magnetically enhanced cancer imaging and targeting in living subjects. ACS Nano 6, 6862–6869 (2012).
Yellen, B. B. et al. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 293, 647–654 (2005).
Polyak, B. et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl Acad. Sci. USA 105, 698–703 (2008).
Xia, N. et al. Combined microfluidic–micromagnetic separation of living cells in continuous flow. Biomed. Micro. 8, 299–308 (2006).
Yavuz, C. T. et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314, 964–967 (2006).
Spivack, D. E., Kelly, P., Gaughan, J. P. & van Bemmelen, P. S. Mapping of superficial extremity veins: normal diameters and trends in a vascular patient-population. Ultrasound Med. Biol. 38, 190–194 (2012).
Thiriet, M. Biology and Mechanics of Blood Flows. CRM Series in Mathematical Physics (Springer, New York, 2008).
Aktas, B. et al. Stem cell and epithelial–mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46 (2009).
Zborowski, M. in Laboratory Techniques in Biochemistry and Molecular Biology Vol. 32, 29–61 (Elsevier, 2007).
Paterlini-Brechot, P. & Benali, N. L. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 253, 180–204 (2007).
Sharma, S. V., Bell, D. W., Settleman, J. & Haber, D. A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7, 169–181 (2007).
Litwin, M. S. & Chapman, K. Physical factors affecting human blood viscosity. J. Surg. Res. 10, 433–436 (1970).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Liu, Y. et al. Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer 13, 202 (2013).
Meng, S. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010).
Dorsey, J. F. et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer 121, 139–149 (2015).
Jain, T. K., Reddy, M. K., Morales, M. A., Leslie-Pelecky, D. L. & Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 5, 316–327 (2008).
Tate, J. A., Petryk, A. A., Giustini, A. J. & Hoopes, P. J. In vivo biodistribution of iron oxide nanoparticles: an overview. Proc. SPIE Int. Soc. Opt. Eng. 7901, 790117 (2011).
Arami, H., Khandhar, A., Liggitt, D. & Krishnan, K. M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44, 8576–8607 (2015).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Coumans, F. & Terstappen, L. in Whole Genome Amplification. Methods and Protocols Vol. 1347 (ed. Kroneis, T.) 263–278 (Humana Press, 2015).
Talasaz, A. H. et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc. Natl Acad. Sci. USA 106, 3970–3975 (2009).
Galanzha, E. I. et al. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 4, 855–860 (2009).
Fonnum, G., Johansson, C., Molteberg, A., Mørup, S. & Aksnes, E. Characterisation of Dynabeads® by magnetization measurements and Mössbauer spectroscopy. J. Magn. Magn. Mater. 293, 41–47 (2005).
Vermesh, O. et al. Dataset for ‘An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo’. figshare https://doi.org/10.6084/m9.figshare.6272414 (2018).
Diaz, L. A. & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014).
Acknowledgements
We thank D. Sze, A. Thakor, M. Mahmoudi, H. Nejadnik, T. Larson, A. de Souza and H. Tom Soh for discussions. We also thank Pork Power Farms for their help in choosing suitable pigs for the study. We would also like to acknowledge the Veterinary Service Center and Animal Diagnostic Laboratory at Stanford. This research was supported by the US National Institutes of Health (NIH) Awards U54CA151459 (Center for Cancer Nanotechnology Excellence and Translation) and R21CA185804 (to S.S.G. and S.X.W.), the Canary Foundation (to S.S.G.), and the Ben and Catherine Ivy Foundation. The authors also acknowledge funding support from the NIH Shared Instrument Grant S10 RR026714.
Author information
Authors and Affiliations
Contributions
O.V., A.A., T.J.G. and S.S.G conceived and designed the research. O.V., A.A. and T.J.G. performed all experiments. O.V., A.A., T.J.G., S.-m.P., C.N.A., E.I.S., S.X.W. and S.S.G analysed the data. T.J.G. and Y.G. performed the computational modelling. Y.M., Y.S., J.K.L, A.G. and K.M. aided with the porcine model. O.V., I.S.A., C.N.A., J.V.-M., E.G. and E.I.S. conducted and analysed the toxicity, biodistribution and pharmacokinetic studies. C.C.O. and H.A. aided with MP characterization. M.H.B. contributed cell culture expertise and reagents. O.V., A.A., T.J.G., S.-m.P. and S.S.G drafted the manuscript with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
O.V., A.A., T.J.G., S.-m.P, and S.S.G. have filed for patent protection for the MagWIRE technology. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
Trajectories and distribution of magnetic particles along the MagWIRE.
Supplementary Video 2
Magnetic particle accumulation on MagWIRE.
Supplementary Video 3
Single-pass method of rapid cell labelling and capture.
Supplementary Video 4
Fluoroscopy of the highly vascularized porcine ear.
Supplementary Video 5
Fluoroscopy of the MagWIRE in a porcine ear vein.
Rights and permissions
About this article
Cite this article
Vermesh, O., Aalipour, A., Ge, T.J. et al. An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo. Nat Biomed Eng 2, 696–705 (2018). https://doi.org/10.1038/s41551-018-0257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0257-3
This article is cited by
-
PD-L1-driven efficient enrichment and elimination of circulating cancer cells by magnetic MoSe2 nanosheet
Nano Research (2024)
-
Analytical device miniaturization for the detection of circulating biomarkers
Nature Reviews Bioengineering (2023)
-
In vivo detection demonstrates circulating tumor cell reduction instead of baseline number has prognostic value in bladder cancer patients receiving neoadjuvant chemotherapy
Cellular Oncology (2023)
-
In vivo detection of circulating tumor cells predicts high-risk features in patients with bladder cancer
Medical Oncology (2023)
-
Where Are We Now and Where Might We Be Headed in Understanding and Managing Brain Metastases in Colorectal Cancer Patients?
Current Treatment Options in Oncology (2022)