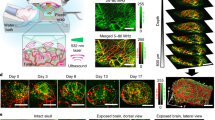
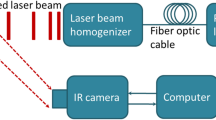
Abstract
Low signal-to-noise ratios and limited imaging depths restrict the ability of optical-imaging modalities to detect and accurately quantify molecular emissions from tissue. Here, by using a scanning external X-ray beam from a clinical linear accelerator to induce Cherenkov excitation of luminescence in tissue, we demonstrate in vivo mapping of the oxygenation of tumours at depths of several millimetres, with submillimetre resolution and nanomolar sensitivity. This was achieved by scanning thin sheets of the X-ray beam orthogonally to the emission-detection plane, and by detecting the signal via a time-gated CCD camera synchronized to the radiation pulse. We also show with experiments using phantoms and with simulations that the performance of Cherenkov-excited luminescence scanned imaging (CELSI) is limited by beam size, scan geometry, probe concentration, radiation dose and tissue depth. CELSI might provide the highest sensitivity and resolution in the optical imaging of molecular tracers in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hoebe, R. A. et al. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat. Biotechnol. 25, 249–253 (2007).
Brismar, H. & Ulfhake, B. Fluorescence lifetime measurements in confocal microscopy of neurons labeled with multiple fluorophores. Nat. Biotechnol. 15, 373–377 (1997).
Nie, S., Chiu, D. T. & Zare, R. N. Probing individual molecules with confocal fluorescence microscopy. Science 266, 1018–1021 (1994).
Zipfel, W. R. et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl Acad. Sci. USA 100, 7075–7080 (2003).
Bjorn, S., Ntziachristos, V. & Schulz, R. Mesoscopic epifluorescence tomography: reconstruction of superficial and deep fluorescence in highly-scattering media. Opt. Express 18, 8422–8429 (2010).
Georgakoudi, I. et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 120, 1620–1629 (2001).
Mittapalli, R. K., Manda, V. K., Bohn, K. A., Adkins, C. E. & Lockman, P. R. Quantitative fluorescence microscopy provides high resolution imaging of passive diffusion and P-gp mediated efflux at the in vivo blood-brain barrier. J. Neurosci. Methods 219, 188–195 (2013).
Leblond, F., Davis, S. C., Valdes, P. A. & Pogue, B. W. Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J. Photochem. Photobiol. B 98, 77–94 (2010).
Pogue, B. W. Optics in the molecular imaging race. Opt. Photon. News 9, 25–31 (2015).
Zhang, R. et al. Cherenkov-excited luminescence scanned imaging. Opt. Lett. 40, 827–830 (2015).
Bruza, P. et al. Light sheet luminescence imaging with Cherenkov excitation in thick scattering media. Opt. Lett. 41, 2986–2989 (2016).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Zhang, R. et al. Oxygen tomography by Cerenkov-excited phosphorescence during external beam irradiation. J. Biomed. Opt. 18, 50503 (2013).
Dothager, R. S., Goiffon, R. J., Jackson, E., Harpstrite, S. & Piwnica-Worms, D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS ONE 5, e13300 (2010).
Thorek, D. L., Ogirala, A., Beattie, B. J. & Grimm, J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat. Med. 19, 1345–1350 (2013).
Axelsson, J., Glaser, A. K., Gladstone, D. J. & Pogue, B. W. Quantitative Cherenkov emission spectroscopy for tissue oxygenation assessment. Opt. Express 20, 5133–5142 (2012).
Jarvis, L. A. et al. Cherenkov video imaging allows for the first visualization of radiation therapy in real time. Int J. Radiat. Oncol. Biol. Phys. 89, 615–622 (2014).
Axelsson, J., Davis, S., Gladstone, D. & Pogue, B. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med. Phys. 38, 4127–4132 (2011).
Demers, J. L., Davis, S. C., Zhang, R., Gladstone, D. J. & Pogue, B. W. Cerenkov excited fluorescence tomography using external beam radiation. Opt. Lett. 38, 1364–1366 (2013).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Keereweer, S. et al. Image-guided surgery in head and neck cancer: current practice and future directions of optical imaging. Head Neck 34, 120–126 (2012).
Mitchell, G. S., Gill, R. K., Boucher, D. L., Li, C. & Cherry, S. R. In vivo Cerenkov luminescence imaging: a new tool for molecular imaging. Phil. Trans. A Math. Phys. Eng. Sci. 369, 4605–4619 (2011).
Das, S., Thorek, D. L. & Grimm, J. Cerenkov imaging. Adv. Cancer Res 124, 213–234 (2014).
Lin, H. et al. Comparison of Cherenkov excited fluorescence and phosphorescence molecular sensing from tissue with external beam irradiation. Phys. Med Biol. 61, 3955–3968 (2016).
Dsouza, A. et al. Cherenkov-excited multi-fluorophore sensing in tissue-simulating phantoms and in vivo. Radiat. Res. (in the press).
Esipova, T. V. et al. Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Anal. Chem. 83, 8756–8765 (2011).
Czupryna, J. et al. Cerenkov-specific contrast agents for detection of pH in vivo. J. Nucl. Med 56, 483–488 (2015).
Holt, R. W. et al. Cherenkov excited phosphorescence-based pO2 estimation during multi-beam radiation therapy: phantom and simulation studies. Phys. Med Biol. 59, 5317–5328 (2014).
Zhang, R. et al. Cerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies. Biomed. Opt. Express 3, 2381–2394 (2012).
Lehmann, S. et al. In vivo fluorescence imaging of the activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin. Cancer Res. 22, 4417–4427 (2016).
Tang, Q. et al. Depth-resolved imaging of colon tumor using optical coherence tomography and fluorescence laminar optical tomography. Biomed. Opt. Express 7, 5218–5232 (2016).
Kepshire, D. S. et al. Imaging of glioma tumor with endogenous fluorescence tomography. J. Biomed. Opt. 14, 030501 (2009).
Dehghani, H. et al. Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction. Commun. Numer. Methods Eng. 25, 711–732 (2008).
Jermyn, M. et al. Fast segmentation and high-quality three-dimensional volume mesh creation from medical images for diffuse optical tomography. J. Biomed. Opt. 18, 86007 (2013).
Acknowledgements
This work has been funded by the Congressionally Directed Medical Research Program for Breast Cancer Research Program, US Army USAMRAA contract W81XWH-16-1-0004 and National Institutes of Health research grants R01 EB024498 and R01 EB018464.
Author information
Authors and Affiliations
Contributions
B.W.P. conceived the study, supervised all aspects of the work and drafted the manuscript; J.F., H.L., P.B., E.P.L., R.Z. and J.R.S. each completed measurements and data analysis as well as designed the experiments, wrote initial parts of the manuscript, and edited the entire manuscript. H.D. and S.C.D. helped design and analyse the tomography work with J.F., and each edited the manuscript. S.A.V. provided the molecular probe, provided advice on experimental design and data analysis and edited the manuscript. D.J.G. and L.A.J. each contributed advice on radiotherapy design and data interpretation, as well as edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, methods, figures, tables and video captions.
Supplementary Video 1
Three-dimensional view of a xenograft tumour imaged by CELSI.
Supplementary Video 2
Real-time scanned acquisition of CELSI data in vivo.
Rights and permissions
About this article
Cite this article
Pogue, B.W., Feng, J., LaRochelle, E.P. et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nat Biomed Eng 2, 254–264 (2018). https://doi.org/10.1038/s41551-018-0220-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0220-3
This article is cited by
-
Review of Tissue Oxygenation Sensing During Radiotherapy Based Upon Cherenkov-Excited Luminescence Imaging
Applied Magnetic Resonance (2021)
-
Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy
Nature Communications (2020)
-
High-resolution Cherenkov tomography in vivo
Nature Biomedical Engineering (2018)