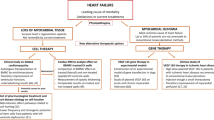
Abstract
The development of cells for regenerative therapy has encountered many pitfalls on its path to clinical translation. In cardiology, clinical studies of heart-targeted cell therapies began two decades ago, yet progress towards reaching an approved product has been slow. In this Perspective, I provide an overview of recent cardiac cell therapies, with a focus on the hurdles limiting the translation of cell products from research laboratories to clinical practice. By focusing on heart failure as a target indication, I argue that strategies for overcoming limitations in clinical translation require an increasing emphasis on mechanism-supported efficacy, rather than on phenomenological observations. As research progresses from cells to paracrine mechanisms to defined factors, identifying those defined factors that are involved in achieving superior therapeutic efficacy will better inform the use of cells as therapeutic candidates. The next generation of cell-free biologics may provide the benefits of cell therapy without the intrinsic limitations of whole-cell products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Roth, G. A. et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017).
Eschenhagen, T. et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017).
Marinescu, K. K., Uriel, N., Mann, D. L. & Burkhoff, D. Left ventricular assist device-induced reverse remodeling: it’s not just about myocardial recovery. Expert Rev. Med. Devices 14, 15–26 (2017).
Menasche, P. et al. Autologous skeletal myoblast transplantation for cardiac insufficiency. First Clin. Case. Arch. Mal. Coeur. Vaiss. 94, 180–182 (2001).
Voronov, R. A. Experimental study of the regenerative potentialities of the cardiac and somatic musculatures. Arkh. Anat. Gistol. Embriol. 69, 35–40 (1975).
Koh, G. Y., Klug, M. G., Soonpaa, M. H. & Field, L. J. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J. Clin. Invest. 92, 1548–1554 (1993).
Taylor, D. A. et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 4, 929–933 (1998).
Menasche, P. et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117, 1189–1200 (2008).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Chien, K. R. Stem cells: lost in translation. Nature 428, 607–608 (2004).
Strauer, B. E. et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 (2002).
Fisher, S. A., Doree, C., Mathur, A., Taggart, D. P. & Martin-Rendon, E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev. 12, CD007888 (2016).
Mathur, A. et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur. Heart J. 38, 2930–2935 (2017).
Jeevanantham, V., Afzal, M. R., Zuba-Surma, E. K. & Dawn, B. Clinical trials of cardiac repair with adult bone marrow-derived cells. Methods Mol. Biol. 1036, 179–205 (2013).
Nowbar, A. N. et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ 348, g2688 (2014).
Wang, L. T. et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci. 23, 76 (2016).
Behfar, A. et al. Guided stem cell cardiopoiesis: discovery and translation. J. Mol. Cell. Cardiol. 45, 523–529 (2008).
Psaltis, P. J. et al. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J. Cell. Physiol. 223, 530–540 (2010).
Golpanian, S., Wolf, A., Hatzistergos, K. E. & Hare, J. M. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol. Rev. 96, 1127–1168 (2016).
Bartunek, J. et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur. J. Heart Fail. 18, 160–168 (2016).
Perin, E. C. et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 117, 576–584 (2015).
Povsic, T. J. et al. The RENEW trial: efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina. JACC Cardiovasc. Interv. 9, 1576–1585 (2016).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Bolli, R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011).
van Berlo, J. H. et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014).
The Lancet Editors. Expression of concern: the SCIPIO trial. Lancet 383, 1279 (2014).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Menasche, P. et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71, 429–438 (2018).
Menasche, P. The future of stem cells: should we keep the “stem” and skip the “cells”? J. Thorac. Cardiovasc. Surg. 152, 345–349 (2016).
The Editor. A futile cycle in cell therapy. Nat. Biotechnol. 35, 291 (2017).
Bolli, R. Repeated cell therapy: a paradigm shift whose time has come. Circ. Res. 120, 1072–1074 (2017).
Tompkins, B. A., Natsumeda, M., Balkan, W. & Hare, J. M. What is the future of cell-based therapy for acute myocardial infarction. Circ. Res. 120, 252–255 (2017).
Quyyumi, A. A. et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ. Res. 120, 324–331 (2017).
Zwetsloot, P. P. et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ. Res. 118, 1223–1232 (2016).
Dodson, B. P. & Levine, A. D. Challenges in the translation and commercialization of cell therapies. BMC Biotechnol. 15, 70 (2015).
Campbell, A. et al. Concise review: process development considerations for cell therapy. Stem Cells Transl. Med 4, 1155–1163 (2015).
Karnieli, O. Cell therapy: early process development and optimization of the manufacturing process are critical to ensure viability of the product, quality, consistency and cost efficiency. J. Commer. Biotechnol. https://doi.org/10.5912/jcb695 (2015).
Szymczak, M. M., Friedman, R. L., Uppoor, R. & Yacobi, A. Detection, measurement, and control in pharma manufacturing. Pharm. Technol. 35, 70–76 (2011).
Bravery, C. A. et al. Potency assay development for cellular therapy products: an ISCT review of the requirements and experiences in the industry. Cytotherapy 15, 9–19 (2013).
Johnston, N., Schenck-Gustafsson, K. & Lagerqvist, B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur. Heart J. 32, 1331–1336 (2011).
Cheng, K. et al. Brief report: mechanism of extravasation of infused stem cells. Stem Cells 30, 2835–2842 (2012).
Suzuki, G. et al. Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PLoS ONE 9, e113009 (2014).
Tseliou, E. et al. Widespread myocardial delivery of heart-derived stem cells by nonocclusive triple-vessel intracoronary infusion in porcine ischemic cardiomyopathy: superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology. PLoS ONE 11, e0144523 (2016).
Golpanian, S. et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl. Med. 5, 186–191 (2016).
Losordo, D. W. et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 109, 428–436 (2011).
Oh, H. Cell therapy trials in congenital heart disease. Circ. Res. 120, 1353–1366 (2017).
Takehara, N. et al. The ALCADIA (Autologous Human Cardiac-Derived Stem Cell to Treat Ischemic Cardiomyopathy) trial. Circulation 126, 2776–2799 (2012).
Fox, I. J. et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 (2014).
Amariglio, N. et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, e1000029 (2009).
Berkowitz, A. L. et al. Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. N. Engl. J. Med. 375, 196–198 (2016).
Thirabanjasak, D., Tantiwongse, K. & Thorner, P. S. Angiomyeloproliferative lesions following autologous stem cell therapy. J. Am. Soc. Nephrol. 21, 1218–1222 (2010).
Malliaras, K. et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125, 100–112 (2012).
Schu, S. et al. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 16, 2094–2103 (2012).
Al-Daccak, R. & Charron, D. Allogenic benefit in stem cell therapy: cardiac repair and regeneration. Tissue Antigens 86, 155–162 (2015).
Smith, R. R., Barile, L., Messina, E. & Marban, E. Stem cells in the heart: what’s the buzz all about? Part 2: arrhythmic risks and clinical studies. Heart Rhythm. 5, 880–887 (2008).
Miyagawa, S. et al. Phase I clinical trial of autologous stem cell–sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart Assoc. 6, e003918 (2017).
Smith, R. R. et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908 (2007).
Sanz-Ruiz, R. & Fernández-Avilés, F. Autologous and allogeneic cardiac stem cell therapy for cardiovascular diseases. Pharmacol. Res. 127, 92–100 (2018).
White, A. J. et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur. Heart J. 34, 68–75 (2013).
Davis, D. R. et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE 4, e7195 (2009).
Makkar, R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012).
Ishigami, S. et al. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ. Res. 120, 1162–1173 (2017).
Tarui, S. et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology (TICAP) trial. J. Thorac. Cardiovasc. Surg. 150, 1198–1207 (2015).
Chimenti, I. et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 106, 971–980 (2010).
Malliaras, K. et al. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol. Med. 6, 760–777 (2014).
Sanz-Ruiz, R. & Fernandez-Aviles, F. Autologous and allogeneic cardiac stem cell therapy for cardiovascular diseases. Pharmacol. Res. 127, 92–100 (2017).
Malliaras, K. et al. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 128, 2764–2775 (2013).
Reich, H. et al. Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J. Heart Lung Transplant. 35, 1348–1357 (2016).
Chakravarty, T. et al. TCT-820 multivessel intracoronary infusion of allogeneic cardiosphere derived cells in dilated cardiomyopathy: long term outcomes of the Dilated Cardiomyopathy Intervention with Allogeneic Myocardially-Regenerative Cells (DYNAMIC trial). J. Am. Coll. Cardiol. 68, B332 (2016).
Ibrahim, A. G., Cheng, K. & Marban, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014).
Ibrahim, A. & Marban, E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu. Rev. Physiol. 78, 67–83 (2016).
Wang, Y. et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 192, 61–69 (2015).
Kervadec, A. et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transplant. 35, 795–807 (2016).
Khan, M. et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117, 52–64 (2015).
Cambier, L. et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 9, 337–352 (2017).
Marban, E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J. Am. Coll. Cardiol. 71, 193–200 (2018).
Aminzadeh, M. A. et al. Exosome-mediated benefits of cell therapy in mouse and human models of Duchenne muscular dystrophy. Stem Cell Rep. 10, 942–955 (2018).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 (2017).
Funakoshi, S. et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 6, 19111 (2016).
Akers, J. C. et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 17, 125–132 (2016).
Bosch, S. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 6, 36162 (2016).
Aminzadeh, M. A. et al. Reversal of cardiac and skeletal manifestations of Duchenne muscular dystrophy by cardiosphere-derived cells and their exosomes in mdx dystrophic mice and in human Duchenne cardiomyocytes. Preprint at https://www.biorxiv.org/content/early/2017/04/20/128900 (2017).
Chen, K. H. et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7, 74537–74556 (2016).
Vandergriff, A. C. et al. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015, 960926 (2015).
Conlan, R. S., Pisano, S., Oliveira, M. I., Ferrari, M. & Mendes Pinto, I. Exosomes as reconfigurable therapeutic systems. Trends Mol. Med. 23, 636–650 (2017).
de Couto, G. et al. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 136, 200–214 (2017).
Aminzadeh, M. A. et al. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur. Heart J. 36, 751–762 (2015).
Tseliou, E. et al. Cardiospheres reverse adverse remodeling in chronic rat myocardial infarction: roles of soluble endoglin and TGF-β signaling. Basic Res. Cardiol. 109, 443 (2014).
Lauden, L. et al. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ. Res. 112, 451–464 (2013).
Marban, E. Breakthroughs in cell therapy for heart disease: focus on cardiosphere-derived cells. Mayo Clin. Proc. 89, 850–858 (2014).
Acknowledgements
This work is supported by grants from the National Institutes of Health, the California Institute for Regenerative Medicine, the United States Department of Defense, and Coalition Duchenne. I thank A. Ibrahim for creating a first draft of Fig. 6a, and L. Marbán for a critical reading of the manuscript and for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.M. holds founder’s equity in, and serves as unpaid scientific advisor to, Capricor Inc.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marbán, E. A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat Biomed Eng 2, 353–361 (2018). https://doi.org/10.1038/s41551-018-0216-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0216-z
This article is cited by
-
Cell Therapy Strategies on Duchenne Muscular Dystrophy: A Systematic Review of Clinical Applications
Stem Cell Reviews and Reports (2024)
-
Can Extracellular Vesicles as Drug Delivery Systems Be a Game Changer in Cardiac Disease?
Pharmaceutical Research (2023)
-
Making fitter cells and tissues
Nature Biomedical Engineering (2022)
-
Functional hydrogels for the treatment of myocardial infarction
NPG Asia Materials (2022)
-
On the cellular origin of cardiosphere-derived cells (CDCs)
Basic Research in Cardiology (2022)