Abstract
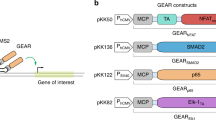
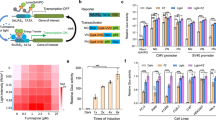
Sophisticated genetic devices can be assembled to reprogram mammalian cell activities using tools from synthetic biology. Here, we demonstrate that a self-adjusting synthetic gene circuit can be designed to sense and reverse the insulin-resistance syndrome in different mouse models. By functionally rewiring the mitogen-activated protein kinase (MAPK) signalling pathway to produce MAPK-mediated activation of a hybrid transcription factor consisting of the tetracycline repressor, TetR, fused to the human ELK1-derived transactivation domain (TetR-Elk1), we assembled a synthetic insulin-sensitive transcription-control device that self-sufficiently distinguished between physiological and increased blood insulin levels and correspondingly fine-tuned the reversible expression of therapeutic transgenes from synthetic TetR-ELK1-specific promoters. In acute experimental hyperinsulinaemia, the synthetic insulin-sensing designer circuit reversed the insulin-resistance syndrome by coordinating expression of the insulin-sensitizing compound adiponectin. Engineering synthetic gene circuits to sense pathologic markers and coordinate the expression of therapeutic transgenes may provide opportunities for future gene- and cell-based treatments of multifactorial metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016).
Johnson, A. M. & Olefsky, J. M. The origins and drivers of insulin resistance. Cell 152, 673–684 (2013).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012).
Prentki, M. & Nolan, C. J. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 (2006).
Christensen, R., Kristensen, P. K., Bartels, E. M., Bliddal, H. & Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713 (2007).
Gloy, V. L. et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Br. Med. J. 347, f5934 (2013).
Loke, Y. K., Kwok, C. S. & Singh, S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. Br. Med. J. 342, d1309 (2011).
Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935 (2001).
Gao, H. et al. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 62, 1338–1344 (2013).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302, 179–188 (2009).
Ziemke, F. & Mantzoros, C. S. Adiponectin in insulin resistance: lessons from translational research. Am. J. Clin. Nutr. 91, 258S–261S (2010).
Slomovic, S., Pardee, K. & Collins, J. J. Synthetic biology devices for in vitro and in vivo diagnostics. Proc. Natl Acad. Sci. USA 112, 14429–14435 (2015).
Bai, P. et al. A synthetic biology-based device prevents liver injury in mice. J. Hepatol. 65, 84–94 (2016).
Ye, H., Aubel, D. & Fussenegger, M. Synthetic mammalian gene circuits for biomedical applications. Curr. Opin. Chem. Biol. 17, 910–917 (2013).
Bacchus, W., Aubel, D. & Fussenegger, M. Biomedically relevant circuit-design strategies in mammalian synthetic biology. Mol. Syst. Biol. 9, 691 (2013).
Ye, H. et al. Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. Proc. Natl Acad. Sci. USA 110, 141–146 (2013).
Kim, T., Folcher, M., Doaud-El Baba, M. & Fussenegger, M. A synthetic erectile optogenetic stimulator enabling blue-light-inducible penile erection. Angew. Chem. 54, 5933–5938 (2015).
Auslander, D. et al. A synthetic multifunctional mammalian pH sensor and CO2 transgene-control device. Mol. Cell 55, 397–408 (2014).
Jacob, K. K., Whittaker, J. & Stanley, F. M. Insulin receptor tyrosine kinase activity and phosphorylation of tyrosines 1162 and 1163 are required for insulin-increased prolactin gene expression. Mol. Cell. Endocrinol. 186, 7–16 (2002).
Siddle, K. Signalling by insulin and IGF receptors: supporting acts and new players. J. Mol. Endocrinol. 47, R1–R10 (2011).
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 (2011).
Keeley, M. B., Busch, J., Singh, R. & Abel, T. TetR hybrid transcription factors report cell signaling and are inhibited by doxycycline. BioTechniques 39, 529–536 (2005).
Ge, H. et al. Generation of novel long-acting globular adiponectin molecules. J. Mol. Biol. 399, 113–119 (2010).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 15, 539–553 (1998).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14, 575–585 (2011).
Weber, W. & Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 13, 21–35 (2012).
Ruder, W. C., Lu, T. & Collins, J. J. Synthetic biology moving into the clinic. Science 333, 1248–1252 (2011).
Wu, C. Y., Rupp, L. J., Roybal, K. T. & Lim, W. A. Synthetic biology approaches to engineer T cells. Curr. Opin. Immunol. 35, 123–130 (2015).
Heng, B. C., Aubel, D. & Fussenegger, M. Prosthetic gene networks as an alternative to standard pharmacotherapies for metabolic disorders. Curr. Opin. Biotech. 35, 37–45 (2015).
Kojima, R., Aubel, D. & Fussenegger, M. Novel theranostic agents for next-generation personalized medicine: small molecules, nanoparticles, and engineered mammalian cells. Curr. Opin. Chem. Biol. 28, 29–38 (2015).
Okada-Iwabu, M. et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 (2013).
Fussenegger, M. et al. Streptogramin-based gene regulation systems for mammalian cells. Nat. Biotechnol. 18, 1203–1208 (2000).
Trounson, A. & DeWitt, N. D. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 17, 194–200 (2016).
Lathuiliere, A., Cosson, S., Lutolf, M. P., Schneider, B. L. & Aebischer, P. A high-capacity cell macroencapsulation system supporting the long-term survival of genetically engineered allogeneic cells. Biomaterials 35, 779–791 (2014).
Simonsen, J. L. et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 20, 592–596 (2002).
Wieland, M., Auslander, D. & Fussenegger, M. Engineering of ribozyme-based riboswitches for mammalian cells. Methods 56, 351–357 (2012).
Mates, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 (2009).
Schlatter, S., Rimann, M., Kelm, J. & Fussenegger, M. SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus alpha-amylase. Gene 282, 19–31 (2002).
Weber, W. et al. Gas-inducible transgene expression in mammalian cells and mice. Nat. Biotechnol. 22, 1440–1444 (2004).
Kemmer, C. et al. A designer network coordinating bovine artificial insemination by ovulation-triggered release of implanted sperms. J. Control. Release 150, 23–29 (2011).
Folcher, M., Xie, M., Spinnler, A. & Fussenegger, M. Synthetic mammalian trigger-controlled bipartite transcription factors. Nucleic Acids Res. 41, e134 (2013).
Acknowledgements
We thank T. Abel for providing the pTetR-ELK1 (MKp37) plasmid, B. Geering for providing human serum from healthy individuals, Y. Lai for providing the DyLight 800-labelled goat anti-mouse IgG, B. M. Lang and L. Scheller for assistance with the statistical analyses and M. Daoud-El Baba for skilful assistance with the animal study. This work was supported by a European Research Council (ERC) advanced grant (no. 321381), the Cantons of Basel and the Swiss Confederation within the INTERREG IV A.20 tri-national research program and the Gutenberg Chair (awarded to M.F.). This work was also supported by the National Key Research and Development Program of China, Stem Cell and Translational Research (no. 2016YFA0100300), the National Natural Science Foundation of China (NSFC; nos 31470834, 31522017 and 31670869), the Science and Technology Commission of Shanghai Municipality (nos 15QA1401500 and 14JC1401700) and Thousand Youth Talents Plan (awarded to H.Y.).
Author information
Authors and Affiliations
Contributions
H.Y., M.X., H.Z. and M.F. designed the project, analysed the results and wrote the manuscript. H.Y., M.X., G.H.E., S.X. and J.Y. performed the experimental work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures and Tables (PDF 1440 kb)
Rights and permissions
About this article
Cite this article
Ye, H., Xie, M., Xue, S. et al. Self-adjusting synthetic gene circuit for correcting insulin resistance. Nat Biomed Eng 1, 0005 (2017). https://doi.org/10.1038/s41551-016-0005
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-016-0005
This article is cited by
-
AAV-delivered muscone-induced transgene system for treating chronic diseases in mice via inhalation
Nature Communications (2024)
-
A programmable protease-based protein secretion platform for therapeutic applications
Nature Chemical Biology (2024)
-
Applications of synthetic biology in medical and pharmaceutical fields
Signal Transduction and Targeted Therapy (2023)
-
A versatile bioelectronic interface programmed for hormone sensing
Nature Communications (2023)
-
Engineering antiviral immune-like systems for autonomous virus detection and inhibition in mice
Nature Communications (2022)