Abstract
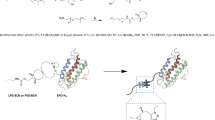
The delivery of therapeutic peptides and proteins is often challenged by short half-lives and the consequent need for frequent injections that limit efficacy, reduce patient compliance and increase treatment cost. Here, we demonstrate that a single subcutaneous injection of site-specific (C-terminal) conjugates of exendin-4 (exendin)—a therapeutic peptide that is clinically used to treat type 2 diabetes mellitus—and poly[oligo(ethylene glycol) methyl ether methacrylate] (POEGMA) with precisely controlled molecular weights lowered blood glucose for up to 120 h in fed mice. Most notably, we show that an exendin-C-POEGMA conjugate with an average of nine side-chain ethylene glycol (EG) repeats exhibits significantly lower reactivity towards patient-derived anti-poly(ethylene glycol) (anti-PEG) antibodies than two US FDA-approved PEGylated drugs, and that reducing the side-chain length to three EG repeats completely eliminates PEG antigenicity without compromising in vivo efficacy. Our findings establish the site-specific conjugation of POEGMA as a next-generation PEGylation technology for improving the pharmacological performance of traditional PEGylated drugs, whose safety and efficacy are hindered by pre-existing anti-PEG antibodies in patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 7, 21–39 (2008).
Craik, D. J., Fairlie, D. P., Liras, S. & Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 81, 136–147 (2013).
Peters, T. J. Serum albumin. Adv. Protein Chem. 37, 161–245 (1985).
Awai, M. & Brown, E. B. Studies of the metabolism of I-131-labeled human transferrin. J. Lab. Clin. Med. 61, 363–396 (1963).
Banga, A. K. Therapeutic Peptides and Proteins: Formulation, Processing, and Delivery Systems (CRC, 2015).
Caliceti, P. & Veronese, F. M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 55, 1261–1277 (2003).
Malik, D. K., Baboota, S., Ahuja, A., Hasan, S. & Ali, J. Recent advances in protein and peptide drug delivery systems. Curr. Drug Deliv. 2, 141–151 (2007).
Werle, M. & Bernkop-Schnurch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30, 351–367 (2006).
Alconcel, S. N. S., Baas, A. S. & Maynard, H. D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2, 1442–1448 (2011).
Nucci, M. L., Shorr, R. G. & Abuchowski, A. The therapeutic value of poly(ethylene glycol)-modified proteins. Adv. Drug Deliv. Rev. 6, 133–151 (1991).
Youn, Y. S., Na, D. H. & Lee, K. C. High-yield production of biologically active mono-PEGylated salmon calcitonin by site-specific PEGylation. J. Control. Release 117, 371–379 (2007).
Gauthier, M. A. & Klok, H. Peptide/protein–polymer conjugates: synthetic strategies and design concepts. Chem. Commun. 2591–2611 (2008).
Qi, Y. & Chilkoti, A. Growing polymers from peptides and proteins: a biomedical perspective. Polym. Chem. 5, 266–276 (2014).
Gaberc-Porekar, V., Zore, I., Podobnik, B. & Menart, V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr. Opin. Drug Discov. Devel. 11, 242–250 (2008).
Veronese, F. M. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 22, 405–417 (2001).
Ganson, N. J., Kelly, S. J., Scarlett, E., Sundy, J. S. & Hershfield, M. S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 8, R12–R22 (2006).
Hershfield, M. S. et al. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 16, R63 (2014).
Armstrong, J. K. et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 110, 103–111 (2007).
Richter, A. W. & Akerblom, E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int. Arch. Allergy Appl. Immunol. 74, 36–39 (1984).
Garay, R. P., El-Gewely, R., Armstrong, J. K., Garratty, G. & Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 9, 1319–1323 (2012).
Ganson, N. J. et al. Pre-existing anti-PEG antibody linked to first-exposure allergic reactions to Pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 137, 1610–1613 (2016).
Qi, Y., Amiram, M., Gao, W., McCafferty, D. G. & Chilkoti, A. Sortase-catalyzed initiator attachment enables high yield growth of a stealth polymer from the C terminus of a protein. Macromol. Rapid Commun. 34, 1256–1260 (2013).
Matyjaszewski, K. & Tsarevsky, N. V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 136, 6513–6533 (2014).
Matyjaszewski, K. & Xia, J. Atom transfer radical polymerization. Chem. Rev. 101, 2921–2990 (2001).
Bontempo, D. & Maynard, H. D. Streptavidin as a macroinitiator for polymerization: in situ protein-polymer conjugate formation. J. Am. Chem. Soc. 127, 6508–6509 (2005).
Gao, W. et al. In situ growth of a stoichiometric PEG-like conjugate at a protein’s N-terminus with significantly improved pharmacokinetics. Proc. Natl Acad. Sci. USA 106, 15231–15236 (2009).
Gao, W., Liu, W., Christensen, T., Zalutsky, M. R. & Chilkoti, A. In situ growth of a PEG-like polymer from the C terminus of an intein fusion protein improves pharmacokinetics and tumor accumulation. Proc. Natl Acad. Sci. USA 107, 16432–16437 (2010).
Peeler, J. C. et al. Genetically encoded initiator for polymer growth from proteins. J. Am. Chem. Soc. 132, 13575–13577 (2010).
Lele, B. S., Murata, H., Matyjaszewski, K. & Russell, A. J. Synthesis of uniform protein-polymer conjugates. Biomacromolecules 6, 3380–3387 (2005).
Ryan, S. M. et al. PK/PD modelling of comb-shaped PEGylated salmon calcitonin conjugates of differing molecular weights. J. Control. Release 149, 126–132 (2011).
Magnusson, J. P., Bersani, S., Salmaso, S., Alexander, C. & Caliceti, P. In situ growth of side-chain PEG polymers from functionalized human growth hormone—a new technique for preparation of enhanced protein-polymer conjugates. Bioconjugate Chem. 21, 671–678 (2010).
Lovshin, J. A. & Drucker, D. J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5, 262–269 (2009).
Boekhorst, J., de Been, M. W., Kleerebezem, M. & Siezen, R. J. Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J. Bacteriol. 187, 4928–4934 (2005).
Meyer, D. E. & Chilkoti, A. Purification of recombinant proteins by fusion with thermally-responsive polypeptide. Nat. Biotechnol. 14, 1112–1115 (1999).
Mao, H., Hart, S. A., Schink, A. & Pollok, B. A. Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc. 126, 2670–2671 (2004).
Jakubowski, W. & Matyjaszewski, K. Activators regenerated by electron transfer for atom-transfer radical polymerization of (meth)acrylates and related block copolymers. Angew. Chem. Int. Ed. 45, 4482–4486 (2006).
Goke, R. et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem . 268, 19650–19655 (1993).
Winzell, M. S. & Ahren, B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, S215–S219 (2004).
Surwit, R. S., Kuhn, C. M., Cochrane, C., McCubbin, J. A. & Feinglos, M. N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163–1167 (1988).
Mack, C. M. et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int. J. Obes. 30, 1332–1340 (2006).
Kanoski, S. E., Rupperecht, L. W., Fortin, S. M., De Jonghe, B. C. & Hayes, M. R. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62, 1916–1927 (2012).
Richter, A. W. & Akerblom, E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 70, 124–131 (1983).
Tsarevsky, N. V., Pintauer, T. & Matyjaszewski, K. Deactivation efficiency and degree of control over polymerization in ATRP in protic solvents. Macromolecules 37, 9768–9778 (2004).
Averick, S. et al. Protein-polymer hybrids: conducting ARGET ATRP from a genetically encoded cleavable ATRP initiator. Eur. Polym. J. 49, 2919–2924 (2013).
Bellucci, J. J., Bhattacharyya, J. & Chilkoti, A. A noncanonical function of sortase enables site-specific conjugation of small molecules to lysine residues in proteins. Angew. Chem. Int. Ed. 54, 441–445 (2015).
Amiram, M., Luginbuhl, K. M., Li, X., Feinglos, M. N. & Chilkoti, A. Injectable protease-operated depots of glucagon-like peptide-1 provide extended and tunable glucose control. Proc. Natl Acad. Sci. USA 110, 2792–2797 (2013).
Schellenberger, V. et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat. Biotechnol. 27, 1186–1188 (2009).
Liao, Y.-D., Jeng, J.-C., Wang, C.-F., Wang, S.-C. & Chang, S.-T. Removal of N-terminal methionine from recombinant proteins by engineered E. coli methionine aminopeptidase. Prot. Sci. 13, 1802–1810 (2004).
McDaniel, J. R., Mackay, J. A., Quiroz, F. G. & Chilkoti, A. Recursive directional ligation by plasmid reconstruction allows rapid and seamless cloning of oligomeric genes. Biomacromolecules 11, 944–952 (2010).
Ilangovan, U., Ton-That, H., Iwahara, J., Schneewind, I. & Clubb, R. T. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus . Proc. Natl Acad. Sci. USA 98, 6056–6061 (2001).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Baggio, L. L., Huang, Q. L., Brown, T. J. & Drucker, D. J. A recombinant human glucagon-like peptide (GLP)-1- albumin protein (Albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53, 2492–2500 (2004).
Goutelle, S. et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 22, 633–648 (2008).
Qi, Y. et al. Dataset for A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. figshare http://dx.doi.org/10.6084/m9.figshare.3976761 (2016).
Zong, Y., Bice, T. W., Ton-That, H., Schneewind, O. & Narayana, S. V. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J. Biol. Chem. 279, 31383–31389 (2004).
Neidigh, J. W., Fesinmeyer, R. M., Prickett, K. S. & Andersen, N. H. Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry 40, 13188–13200 (2001).
Acknowledgements
The authors thank D.M. Gooden at the Duke Small Molecule Synthesis Facility for the synthesis of AEBMP, E.J. Soderblom at the Duke Proteomics Facility for conducting LC/MS-MS, G. Dubay at the Duke Chemistry Mass Spectrometry Facility for LC/ESI-MS support and M.N. Feinglos for discussion of the in vivo results. BHK cells expressing GLP-1R were a gift from the Drucker group (University of Toronto, Canada). This work was supported by the National Institutes of Health (R01-DK092665 to A.C.).
Author information
Authors and Affiliations
Contributions
Y.Q. and A.C. conceived and designed the research. Y.Q., A.S., N.J.G., X.L, I.O. and W.L. performed the experiments. A.S. and K.M. provided technical expertise in polymer chemistry. K.M.L. and W.L. contributed to the design of the in vivo studies. N.J.G. and M.S.H. provided materials and technical expertise for the antigenicity studies. Y.Q., N.J.G., K.M.L., M.S.H., K.M. and A.C. analysed and interpreted the results. Y.Q. and A.C. wrote the manuscript and A.S., N.J.G., K.M.L., M.S.H. and K.M. edited the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.C. and Y.Q. have a pending patent on the sortase-catalysed C-terminal polymer conjugation technology (WO 2014194244 A1). M.S.H. is a co-inventor of Pegloticase (Krystexxa) and receives royalties from sales of Pegloticase, along with his employer, Duke University. The results reported in this paper form the basis of US provisional patent applications (62/270,401; 62/329,800; 62/310,534; 62/407,403) filled by A.C., Y.Q., M.S.H. and N.J.G. through the Duke University Office of Licensing & Ventures.
Supplementary information
Supplementary information
Supplementary methods, figures and tables (PDF 1731 kb)
Rights and permissions
About this article
Cite this article
Qi, Y., Simakova, A., Ganson, N. et al. A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. Nat Biomed Eng 1, 0002 (2017). https://doi.org/10.1038/s41551-016-0002
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-016-0002
This article is cited by
-
Biomaterial-based antimicrobial therapies for the treatment of bacterial infections
Nature Reviews Materials (2021)
-
A ribonucleoprotein octamer for targeted siRNA delivery
Nature Biomedical Engineering (2018)
-
Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy
Nature Communications (2017)